A novel antibacterial hydrogel containing aminophylline as a versatile platform for neural differentiation of hWJMSCs through the CREB pathway
- PMID: 40413259
- PMCID: PMC12103626
- DOI: 10.1038/s41598-025-02584-w
A novel antibacterial hydrogel containing aminophylline as a versatile platform for neural differentiation of hWJMSCs through the CREB pathway
Abstract
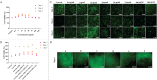
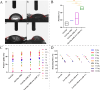
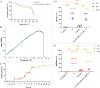
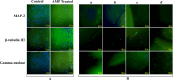
This study aims to develop a novel antibacterial hydrogel scaffold composed of gelatin (Gel), amniotic membrane extract (AME), and aminophylline (AMP) for neural regeneration. We investigate its ability to sustain AMP release, inhibit bacterial growth, and promote neural differentiation of human Wharton's jelly mesenchymal stem cells (hWJMSCs) via the CREB pathway, addressing unmet needs in neural tissue engineering. The composite hydrogels were synthesized and characterized using various methods and techniques, including X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), Fourier transform infrared spectroscopy (FTIR), porosity, contact angle, water uptake, thermogravimetric analysis (TGA), biodegradation, tensile strength, drug release, and antibacterial activity. Biocompatibility tests (MTT assay, AO/EB staining) confirmed > 95% viability of hWJMSCs over six days and their differentiation to the neural cells was analyzed through immunocytochemistry (ICC) staining and real-time reverse transcription-polymerase chain reaction (RT-PCR) at different time points. The results demonstrate the successful synthesis of porous hydrogels with desirable properties, including hydrophilicity, thermal stability, biodegradability, and mechanical strength. The hydrogels support the sustained release of AMP (53.18% over 336 h) and exhibit antibacterial activity against Pseudomonas aeruginosa (90.52 ± 0.26%) and Staphylococcus aureus (93.06 ± 0.34%) due to the presence of penicillin and streptomycin (P-S) antibiotics. The biocompatibility results show that the hydrogels do not have a cytotoxic effect on the viability of human WJMSCs. The neural differentiation of human WJMSCs seeded on surface hydrogels was confirmed by evaluating specific neural markers at both protein and gene levels. In conclusion, the new antibacterial gel-based hydrogel can support the release of AMP and after further evaluation, can be introduced as a new candidate for neural repair applications.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval and consent to participate: This project was evaluated by the Research Ethics Committees of the Islamic Azad Tehran Medical Sciences University - Pharmacy and Pharmaceutical Branches Faculty and has Approval ID: IR.IAU.PS.REC.1402.301.
Figures


Similar articles
-
Auricular cartilage regeneration using chondroitin sulfate-based hydrogel with mesenchymal stem cells in rabbits.Artif Organs. 2024 Oct;48(10):1100-1111. doi: 10.1111/aor.14807. Epub 2024 Jul 19. Artif Organs. 2024. PMID: 39031117
-
A novel hydrogel scaffold contained bioactive glass nanowhisker (BGnW) for osteogenic differentiation of human mesenchymal stem cells (hMSCs) in vitro.Int J Biol Macromol. 2021 Mar 31;174:562-572. doi: 10.1016/j.ijbiomac.2021.01.002. Epub 2021 Jan 9. Int J Biol Macromol. 2021. PMID: 33434552
-
Proliferation and differentiation of Wharton's jelly-derived mesenchymal stem cells on prgf-treated hydrogel scaffold.Regen Med. 2024 Nov;19(11):549-560. doi: 10.1080/17460751.2024.2427513. Epub 2024 Nov 18. Regen Med. 2024. PMID: 39558722
-
Gentamycin-loaded halloysite-based hydrogel nanocomposites for bone tissue regeneration: fabrication, evaluation of the antibacterial activity and cell response.Biomed Mater. 2022 Oct 14;17(6). doi: 10.1088/1748-605X/ac94ad. Biomed Mater. 2022. PMID: 36150376
-
Melissa officinalis extract nanoemulsion, Caffeic acid and Quercetin as a novel inducer for investigating neural differentiation of human Wharton's jelly mesenchymal stem cells.Tissue Cell. 2025 Aug;95:102815. doi: 10.1016/j.tice.2025.102815. Epub 2025 Mar 7. Tissue Cell. 2025. PMID: 40073469
References
-
- Sabouni, N. et al. Role of curcumin and its nanoformulations in the treatment of neurological diseases through the effects on stem cells. J. Drug Target31, 243–260 (2023). - PubMed
-
- Shariati, A. et al. Mesenchymal stromal cells (MSCs) for neurodegenerative disease: a promising frontier. Eur. J. Cell Biol.99, 151097 (2020). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

