Low-intensity laser exposure enhances rice (Oryza sativa L.) growth through physio-biochemical regulation, transcriptional modulation, and microbiome alteration
- PMID: 40413401
- PMCID: PMC12102851
- DOI: 10.1186/s12870-025-06754-w
Low-intensity laser exposure enhances rice (Oryza sativa L.) growth through physio-biochemical regulation, transcriptional modulation, and microbiome alteration
Abstract
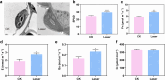
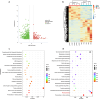
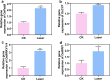
Environmental stressors significantly impact plant growth and agricultural productivity, necessitating innovative approaches to enhance crop resilience and yield. While high-intensity laser applications in agriculture have traditionally been limited to destructive purposes due to their harmful effects on plant growth, the emergence of low-intensity laser technology presents new opportunities for crop improvement. However, the molecular mechanisms underlying the beneficial effects of low-intensity laser treatment remain largely unexplored. This study investigated the effects of low-intensity laser treatment on rice seedling growth, physiological and molecular responses, and rhizosphere microbial communities. Low-intensity laser treatment (2 µmol/m²/s PPFD) significantly enhanced root and shoot growth, enhanced biomass accumulation, and improved yield parameters, with a 16.8% increase in effective panicles and 9.01% higher yield per plant. Physiological analyses revealed elevated antioxidant enzyme activities (POD and SOD) and reduced ROS levels in treated plants. Transmission electron microscopy showed improved chloroplast structure, correlating with enhanced photosynthetic efficiency. Transcriptomic analysis identified 623 differentially expressed genes, with significant enrichment in pathways related to photosynthesis, carbon metabolism, and hormone signaling. Notably upregulation was observed in photosynthesis-related genes (OsPsbB and OsCYF) and hormone signaling genes (OsWRKY114 and OsWRI1). Additionally, 16S rRNA sequencing revealed significant restructuring of rhizosphere bacterial communities in laser-treated plants, with enrichment of beneficial genera including Pseudomonas and Enterobacter. These findings establish low-intensity laser treatment as a promising tool for enhancing rice productivity through coordinated regulation of photosynthetic efficiency, stress responses, and beneficial microbiome interactions.
Keywords: Antioxidant enzymes; Laser; Microbiome; Photosynthesis ROS; Rice.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Two rice cultivars recruit different rhizospheric bacteria to promote aboveground regrowth after mechanical defoliation.Microbiol Spectr. 2025 Jan 7;13(1):e0125424. doi: 10.1128/spectrum.01254-24. Epub 2024 Dec 9. Microbiol Spectr. 2025. PMID: 39651854 Free PMC article.
-
The knockdown of chloroplastic ascorbate peroxidases reveals its regulatory role in the photosynthesis and protection under photo-oxidative stress in rice.Plant Sci. 2014 Jan;214:74-87. doi: 10.1016/j.plantsci.2013.10.001. Epub 2013 Oct 8. Plant Sci. 2014. PMID: 24268165
-
Application of rice (Oryza sativa L.) root endophytic diazotrophic Azotobacter sp. strain Avi2 (MCC 3432) can increase rice yield under green house and field condition.Microbiol Res. 2019 Feb;219:56-65. doi: 10.1016/j.micres.2018.11.004. Epub 2018 Nov 23. Microbiol Res. 2019. PMID: 30642467
-
Microbial Contributions for Rice Production: From Conventional Crop Management to the Use of 'Omics' Technologies.Int J Mol Sci. 2022 Jan 10;23(2):737. doi: 10.3390/ijms23020737. Int J Mol Sci. 2022. PMID: 35054923 Free PMC article. Review.
-
Rhizosphere Microbiome and Functioning in Alternative Rice Cropping Methods: A Critical Review for Rice Sustainability.Front Biosci (Elite Ed). 2025 Feb 14;17(1):25926. doi: 10.31083/FBE25926. Front Biosci (Elite Ed). 2025. PMID: 40150981 Review.
References
-
- Raza A, Ashraf F, Zou X, Zhang X, Tosif H. Plant adaptation and tolerance to environmental stresses: mechanisms and perspectives. Plant ecophysiology and adaptation under climate change: mechanisms and perspectives I: General consequences and plant responses 2020:117–145.
-
- Demmig-Adams B, Polutchko SK, Stewart JJ, Adams WW III. History of excess-light exposure modulates extent and kinetics of fast-acting non-photochemical energy dissipation. Plant Physiol Rep. 2022;27(4):560–72.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

