Glioneuronal tumors PATZ1-fused: clinico-molecular and DNA methylation signatures for a variety of morphological and radiological profiles
- PMID: 40413488
- PMCID: PMC12102922
- DOI: 10.1186/s40478-025-02037-5
Glioneuronal tumors PATZ1-fused: clinico-molecular and DNA methylation signatures for a variety of morphological and radiological profiles
Abstract
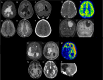
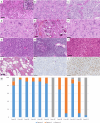
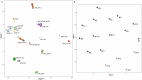
The neuroepithelial tumor, PATZ1-fused (NET-PATZ1), has been recently isolated as a distinct methylation class by DNA-methylation profiling and is characterized by recurrent PATZ1 fusions, in association with the EWSR1 or MN1 genes and a chromosome 22 chromothripsis. The clinical phenotype is mainly pediatric and features circumscribed supratentorial tumors. However, the histopathology is vastly heterogeneous (glial, glioneuronal, sarcomatous, multiphenotypic) and a cell of origin has not yet been identified, explaining the previsionary imprecise terminology of "NET". Moreover, extra-central nervous system (CNS) sarcomas also harboring the EWSR1::PATZ1 fusion have been reported and added to the current World Health Organization (WHO) Classification of Soft Tissue and Bone Tumors, in the chapter on undifferentiated small round cell sarcomas. However, their relationship to their CNS counterparts has not yet been studied. Herein, we analyzed a cohort of twelve CNS tumors with PATZ1 fusions in terms of clinical presentation, radiology, histopathology, immunohistochemistry, ultrastructure and DNA-methylation profiling and compared them to five extra-CNS sarcomas-PATZ1. Based on the reported GATA2 overexpression in NET-PATZ1, we also studied the potential interest of GATA2 immunoexpression as a diagnostic tool. We confirmed their distinct molecular characteristics and clinical phenotype but evidenced a morphological intratumoral heterogeneity with three recurrent morphological patterns (oligodendroglial-like, pleomorphic xanthoastrocytoma-like and spindle cells). Despite the unusual spindle and proliferative component in a CD34 + glioneuronal tumor (using electronic microscopy), these tumors present a favorable prognosis. Their histopathological features were all clearly distinct from their soft tissue counterparts. GATA2 immunostaining is highly specific for CNS tumors PATZ1-fused, but its sensitivity is perfectible and further studies are needed to confirm its use as a diagnostic tool. To conclude, our work highlights that CNS tumors, PATZ1-fused seem to represent a novel pediatric glioneuronal tumor type exhibiting a polymorphous morphology and provides new support for its addition as a provisional emerging pediatric circumscribed glioneuronal tumor type, low grade.
Keywords: DNA-methylation; EWSR1::PATZ1; Glioneuronal tumor; MN1::PATZ1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by GHU Paris Psychiatry and Neurosciences, Sainte-Anne Hospital’s local ethic committee. Consent for publication: The patient signed informed consent forms before treatment was started. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
CIC/ATXN1-rearranged tumors in the central nervous system are mainly represented by sarcomas: A comprehensive clinicopathological and epigenetic series.Brain Pathol. 2025 Mar;35(2):e13303. doi: 10.1111/bpa.13303. Epub 2024 Oct 23. Brain Pathol. 2025. PMID: 39442927 Free PMC article.
-
Neuroepithelial tumors of the central nervous system with EWSR1::PATZ1 fusion: a case report and literature review.Front Oncol. 2025 Jun 12;15:1604479. doi: 10.3389/fonc.2025.1604479. eCollection 2025. Front Oncol. 2025. PMID: 40575153 Free PMC article.
-
PATZ1 fusions define a novel molecularly distinct neuroepithelial tumor entity with a broad histological spectrum.Acta Neuropathol. 2021 Nov;142(5):841-857. doi: 10.1007/s00401-021-02354-8. Epub 2021 Aug 21. Acta Neuropathol. 2021. PMID: 34417833 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Alhalabi KT, Stichel D, Sievers P, Peterziel H, Sommerkamp AC, Sturm D, Wittmann A, Sill M, Jäger N, Beck P, Pajtler KW, Snuderl M, Jour G, Delorenzo M, Martin AM, Levy A, Dalvi N, Hansford JR, Gottardo NG, Uro-Coste E, Maurage C-A, Godfraind C, Vandenbos F, Pietsch T, Kramm C, Filippidou M, Kattamis A, Jones C, Øra I, Mikkelsen TS, Zapotocky M, Sumerauer D, Scheie D, McCabe M, Wesseling P, Tops BBJ, Kranendonk MEG, Karajannis MA, Bouvier N, Papaemmanuil E, Dohmen H, Acker T, von Hoff K, Schmid S, Miele E, Filipski K, Kitanovski L, Krskova L, Gojo J, Haberler C, Alvaro F, Ecker J, Selt F, Milde T, Witt O, Oehme I, Kool M, von Deimling A, Korshunov A, Pfister SM, Sahm F, Jones DTW (2021) PATZ1 fusions define a novel molecularly distinct neuroepithelial tumor entity with a broad histological spectrum. Acta Neuropathol (Berl) 142:841–857. 10.1007/s00401-021-02354-8 - PMC - PubMed
-
- Al-Obaidy KI, Bridge JA, Cheng L, Sumegi J, Reuter VE, Benayed R, Hameed M, Williamson SR, Hes O, Alruwaii FI, Segal JP, Wanjari P, Idrees MT, Nassiri M, Eble JN, Grignon DJ (2021) EWSR1-PATZ1 fusion renal cell carcinoma: a recurrent gene fusion characterizing thyroid-like follicular renal cell carcinoma. Mod Pathol Off J U S Can Acad Pathol Inc 34:1921–1934. 10.1038/s41379-021-00833-7 - PubMed
-
- Watson S, Perrin V, Guillemot D, Reynaud S, Coindre J-M, Karanian M, Guinebretière J-M, Freneaux P, Le Loarer F, Bouvet M, Galmiche-Rolland L, Larousserie F, Longchampt E, Ranchere-Vince D, Pierron G, Delattre O, Tirode F (2018) Transcriptomic definition of molecular subgroups of small round cell sarcomas. J Pathol 245:29–40. 10.1002/path.5053 - PubMed
-
- Fontaine A, Basset L, Milin S, Argentin J, Uro-Coste E, Rousseau A (2025) [Neuroepithelial tumor with PATZ1 fusion - case report and focus on an ill-defined entity]. Ann Pathol 45:92–96. 10.1016/j.annpat.2024.01.002 - PubMed
-
- Siegfried A, Rousseau A, Maurage C-A, Pericart S, Nicaise Y, Escudie F, Grand D, Delrieu A, Gomez-Brouchet A, Le Guellec S, Franchet C, Boetto S, Vinchon M, Sol J-C, Roux F-E, Rigau V, Bertozzi A-I, Jones DTW, Figarella-Branger D, Uro-Coste E (2019) EWSR1-PATZ1 gene fusion May define a new glioneuronal tumor entity. Brain Pathol Zurich Switz 29:53–62. 10.1111/bpa.12619 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

