Biological function of RNA-binding proteins in myocardial infarction: a potential emerging therapeutic limelight
- PMID: 40413549
- PMCID: PMC12102849
- DOI: 10.1186/s13578-025-01408-8
Biological function of RNA-binding proteins in myocardial infarction: a potential emerging therapeutic limelight
Abstract
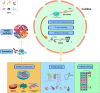
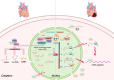
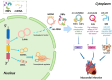
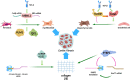
Myocardial infarction (MI) is currently one of the most fatal cardiovascular diseases worldwide. The screening, treatment, and prognosis of MI are top priorities for cardiovascular centers globally due to its characteristic occult onset, high lethality, and poor prognosis. MI is caused by coronary artery occlusion induced by coronary atherosclerotic plaque blockage or other factors, leading to ischemic necrosis and apoptosis of cardiomyocytes. Although significant advancements have been made in the study of cardiomyocytes at the cellular and molecular levels, RNA-binding proteins (RBPs) have not been extensively explored in the context of MI. RBPs, as key regulators coordinating cell differentiation and tissue homeostasis, exhibit specific functions in gene transcription, RNA modification and processing, and post-transcriptional gene expression. By binding to their target RNA, RBPs coordinate various RNA dynamics, including cellular metabolism, subcellular localization, and translation efficiency, thereby controlling the expression of encoded proteins. Classical RBPs, including HuR, hnRNPs, and RBM family molecules, have been identified as critical regulators in myocardial hypoxia, oxidative stress, pro-inflammatory responses, and fibrotic repair. These RBPs exert their effects by modulating key pathophysiological pathways in MI, thereby influencing specific cardiac outcomes. Additionally, specific RBPs, such as QKI and fused in sarcoma (FUS), are implicated in the apoptotic pathways activated during MI. This apoptotic pathway represents a significant molecular phenotype in MI, offering novel perspectives and insights for mitigating cardiomyocyte apoptosis and attenuating the progression of MI. Therefore, this review systematically summarizes the role of RBPs in the main pathophysiological stages of MI and explores their potential therapeutic prospects.
Keywords: Alternative splicing; Myocardial infarction; Post-transcriptional modification; RNA-binding proteins.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures


Similar articles
-
Role of RNA-binding Proteins in Regulating Cell Adhesion and Progression of the Atherosclerotic Plaque and Plaque Erosion.Curr Atheroscler Rep. 2024 Nov 22;27(1):8. doi: 10.1007/s11883-024-01250-2. Curr Atheroscler Rep. 2024. PMID: 39576410 Review.
-
The Biological Mechanisms and Clinical Roles of RNA-Binding Proteins in Cardiovascular Diseases.Biomolecules. 2024 Aug 25;14(9):1056. doi: 10.3390/biom14091056. Biomolecules. 2024. PMID: 39334823 Free PMC article. Review.
-
Emerging roles of circRNAs in the pathological process of myocardial infarction.Mol Ther Nucleic Acids. 2021 Oct 8;26:828-848. doi: 10.1016/j.omtn.2021.10.002. eCollection 2021 Dec 3. Mol Ther Nucleic Acids. 2021. PMID: 34729251 Free PMC article. Review.
-
Hu antigen R (HuR) is a positive regulator of the RNA-binding proteins TDP-43 and FUS/TLS: implications for amyotrophic lateral sclerosis.J Biol Chem. 2014 Nov 14;289(46):31792-31804. doi: 10.1074/jbc.M114.573246. Epub 2014 Sep 19. J Biol Chem. 2014. PMID: 25239623 Free PMC article.
-
Long noncoding RNA Meg3 regulates cardiomyocyte apoptosis in myocardial infarction.Gene Ther. 2018 Dec;25(8):511-523. doi: 10.1038/s41434-018-0045-4. Epub 2018 Oct 4. Gene Ther. 2018. PMID: 30287867
References
-
- Martin SS, Aday AW, Allen NB, Almarzooq ZI, Anderson CAM, Arora P, et al. 2025 heart disease and stroke statistics: A report of US and global data from the American heart association. Circulation. 2025;151:e41–660. - PubMed
-
- Kloner RA, Dai W, Hale SL, Shi J. Approaches to improving cardiac structure and function during and after an acute myocardial infarction: acute and chronic phases. J Cardiovasc Pharmacol Therap. 2016;21:363–7. - PubMed
-
- Gulati R, Behfar A, Narula J, Kanwar A, Lerman A, Cooper L, et al. Acute myocardial infarction in young individuals. Mayo Clin Proc. 2020;95:136–56. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

