Bacteroides uniformis-generated hexadecanedioic acid ameliorates metabolic-associated fatty liver disease
- PMID: 40413726
- PMCID: PMC12118425
- DOI: 10.1080/19490976.2025.2508433
Bacteroides uniformis-generated hexadecanedioic acid ameliorates metabolic-associated fatty liver disease
Abstract
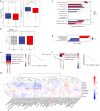
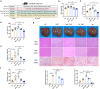
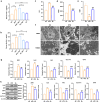
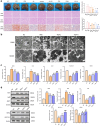
Gut microbiota exerts a pivotal influence on the development of Metabolic Associated Fatty Liver Disease (MAFLD), although the specific contributions of individual bacterial strains and their metabolites remain poorly defined. We conducted stool shotgun metagenomic sequencing and plasma untargeted metabolomics in a large prospective cohort comprising 120 MAFLD patients and 120 matched healthy controls. The mechanisms and microbial-derived metabolites involved in MAFLD were further investigated through multi-omics analyses in vitro and in vivo. Distinct differences were identified in both the microbial community structure and metabolomic profiles between MAFLD patients and healthy controls. Bacteroides uniformis (B. uniformis) was the most significantly depleted species in MAFLD and negatively correlated with hepatic steatosis and BMI. MAFLD was characterized by marked disruptions in fatty acid and amino acid metabolism. Combined analysis of metabolomic and metagenomic data achieved high diagnostic accuracy for MAFLD and hepatic steatosis severity (AUC = 0.93). Transplantation of fecal microbiota from MAFLD subjects into ABX mice led to the onset of MAFLD-like symptoms, whereas B. uniformis administration alleviate disease progression by inhibiting intestinal fat absorption, FFA from eWAT influx into liver via the gut-liver axis, and IRE1α-XBP1s-mediated flipogenesis and ferroptosis, as confirmed by hepatic transcriptomic and proteomic analyses. Hexadecanedioic acid (HDA), potentially identified as a key metabolite produced by B. uniformis, ameliorated MAFLD symptoms. Mechanistically, B. uniformis-derived HDA also inhibited fat absorption and transported, and entered the liver via the portal vein to suppress IRE1α-XBP1s-mediated flipogenesis and ferroptosis. B. uniformis and its potential putative metabolite HDA may contribute to MAFLD progression modulation, through regulation of the IRE1α-XBP1s axis. This study provides new insights into the gut-liver axis in MAFLD and offers promising therapeutic targets based on specific microbes and their metabolites.
Keywords: Bacteroides uniformis; gut microbiota; hexadecanedioic acid; lipid metabolites; metabolic associated fatty liver disease; metabolomics.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, Fujii H, Wu Y, Kam LY, Ji F, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–398. doi: 10.1016/S2468-1253(19)30039-1. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
