The dynamics of stimulus selection in the nucleus isthmi pars magnocellularis of avian midbrain network
- PMID: 40414967
- PMCID: PMC12104471
- DOI: 10.1038/s41598-025-02255-w
The dynamics of stimulus selection in the nucleus isthmi pars magnocellularis of avian midbrain network
Abstract
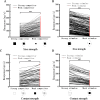
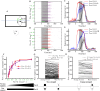
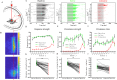
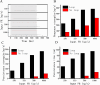
The nucleus isthmi pars magnocellularis (Imc) serves as a critical node in the avian midbrain network for encoding stimulus salience and selection. While reciprocal inhibitory projections among Imc neurons (inhibitory loop) are known to govern stimulus selection, existing studies have predominantly focused on stimulus selection under stimuli of constant relative intensity. However, animals typically encounter complex and changeable visual scenes. Thus, how Imc neurons represent stimulus selection under varying relative stimulus intensities remains unclear. Here, we examined the dynamics of stimulus selection by in vivo recording of Imc neurons' responses to spatiotemporally successive visual stimuli divided into two segments: the previous stimulus and the post stimulus. Our data demonstrate that Imc neurons can encode sensory memory of the previous stimulus, which modulates competition and salience representation in the post stimulus. This history-dependent modulation is also manifested in persistent neural activity after stimulus cessation. We identified, through neural tracing, focal inactivation, and computational modeling experiments, projections from the nucleus isthmi pars parvocellularis (Ipc) to "shepherd's crook" (Shc) neurons, which could be either direct or indirect. These projections enhance Imc neurons' responses and persistent neural activity after stimulus cessation. This connectivity supports a Shc-Ipc-Shc excitatory loop in the midbrain network. The coexistence of excitatory and inhibitory loops provides a neural substrate for continuous attractor network models, a proposed framework for neural information representation. This study also offers a potential explanation for how animals maintain short-term attention to targets in complex and changeable environments.
Keywords: Attractor network; Dynamics; Excitatory loop; Sensory memory; Stimulus competition.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Spatially reciprocal inhibition of inhibition within a stimulus selection network in the avian midbrain.PLoS One. 2014 Jan 21;9(1):e85865. doi: 10.1371/journal.pone.0085865. eCollection 2014. PLoS One. 2014. PMID: 24465755 Free PMC article.
-
Superimposed Inhibitory Surrounds Underlying Saliency-Based Stimulus Selection in Avian Midbrain Isthmi Pars Magnocellularis.Integr Zool. 2025 Feb 10. doi: 10.1111/1749-4877.12957. Online ahead of print. Integr Zool. 2025. PMID: 39929719
-
Spatial Dependence of Stimulus Competition in the Avian Nucleus Isthmi Pars Magnocellularis.Brain Behav Evol. 2019;93(2-3):137-151. doi: 10.1159/000500192. Epub 2019 Aug 15. Brain Behav Evol. 2019. PMID: 31416080 Free PMC article.
-
The role of a midbrain network in competitive stimulus selection.Curr Opin Neurobiol. 2011 Aug;21(4):653-60. doi: 10.1016/j.conb.2011.05.024. Epub 2011 Jun 21. Curr Opin Neurobiol. 2011. PMID: 21696945 Free PMC article. Review.
-
Performance of a Computational Model of the Mammalian Olfactory System.In: Persaud KC, Marco S, Gutiérrez-Gálvez A, editors. Neuromorphic Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2013. Chapter 6. In: Persaud KC, Marco S, Gutiérrez-Gálvez A, editors. Neuromorphic Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2013. Chapter 6. PMID: 26042330 Free Books & Documents. Review.
References
-
- Graybiel, A. M. A satellite system of the superior colliculus: the parabigeminal nucleus and its projections to the superficial collicular layers. Brain Res.145 (2), 365–374 (1978). - PubMed
-
- Sereno, M. I. & Ulinski, P. S. Caudal topographic nucleus Isthmi and the rostral nontopographic nucleus Isthmi in the turtle, Pseudemys scripta. J. Comp. Neurol.261 (3), 319–346 (1987). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

