Targeting of the IL-33/Wnt axis restricts breast cancer stemness and metastasis
- PMID: 40414980
- PMCID: PMC12104483
- DOI: 10.1038/s41598-025-03260-9
Targeting of the IL-33/Wnt axis restricts breast cancer stemness and metastasis
Abstract
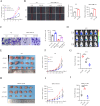
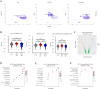
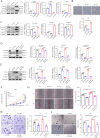
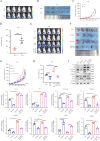
Interleukin-33 (IL-33) plays multifaceted roles in tumor progression, but its autocrine regulation of breast cancer stemness and metastasis via the Wnt pathway remains unclear. Here, we investigated the IL-33/ST2 axis in breast cancer using CRISPR/Cas9, single-cell RNA sequencing, and murine models (orthotopic 4T1 and spontaneous MMTV-PyMT). Elevated IL-33 levels correlated with aggressive subtypes and poor prognosis. IL-33 overexpression enhanced proliferation, migration, and cancer stem cell (CSC) marker expression (CD44, ALDH1) in 4T1 and MDA-MB-231 cells, whereas ST2 knockdown via CRISPR or adeno-associated virus (AAV) attenuated tumor growth and metastasis in vivo, reducing CSC frequency. Mechanistically, IL-33 activated Wnt/β-catenin signaling to promote stemness, which was reversed by the Wnt inhibitor XAV-939. Single-cell analysis revealed that IL-33 overexpression skewed the immune microenvironment toward immunosuppression, while ST2 knockdown restored antitumor immunity. Our findings establish an IL-33-Wnt axis as a critical driver of breast cancer aggressiveness and propose AAV-mediated ST2 silencing as a novel therapeutic strategy. Targeting this axis may offer dual benefits by suppressing stemness and enhancing immune surveillance, warranting clinical exploration for advanced breast cancer.
Keywords: Breast cancer stem cells; IL-33; Immunotherapy; ST2 knockdown; Wnt pathway.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
A synergistic partnership between IL-33/ST2 and Wnt pathway through Bcl-xL drives gastric cancer stemness and metastasis.Oncogene. 2023 Feb;42(7):501-515. doi: 10.1038/s41388-022-02575-5. Epub 2022 Dec 16. Oncogene. 2023. PMID: 36526851
-
The Interleukin-33/ST2 axis promotes glioma mesenchymal transition, stemness and TMZ resistance via JNK activation.Aging (Albany NY). 2020 Jan 29;12(2):1685-1703. doi: 10.18632/aging.102707. Epub 2020 Jan 29. Aging (Albany NY). 2020. PMID: 32003751 Free PMC article.
-
Long non-coding RNA LUCAT1/miR-5582-3p/TCF7L2 axis regulates breast cancer stemness via Wnt/β-catenin pathway.J Exp Clin Cancer Res. 2019 Jul 12;38(1):305. doi: 10.1186/s13046-019-1315-8. J Exp Clin Cancer Res. 2019. PMID: 31300015 Free PMC article.
-
LGR4 modulates breast cancer initiation, metastasis, and cancer stem cells.FASEB J. 2018 May;32(5):2422-2437. doi: 10.1096/fj.201700897R. Epub 2017 Dec 21. FASEB J. 2018. PMID: 29269400 Free PMC article.
-
Interleukin-33/ST2 axis promotes epithelial cell transformation and breast tumorigenesis via upregulation of COT activity.Oncogene. 2015 Sep 17;34(38):4928-38. doi: 10.1038/onc.2014.418. Epub 2014 Dec 22. Oncogene. 2015. PMID: 25531326
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

