Enteral immunization with live bacteria reprograms innate immune cells and protects neonatal foals from pneumonia
- PMID: 40415003
- PMCID: PMC12104368
- DOI: 10.1038/s41598-025-02060-5
Enteral immunization with live bacteria reprograms innate immune cells and protects neonatal foals from pneumonia
Abstract
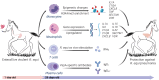
Using a horse foal model, we show that enteral immunization of newborn foals with Rhodococcus equi overcomes neonatal vaccination challenges by reprogramming innate immune responses, inducing R. equi-specific adaptive humoral and cell-mediated immune responses and protecting foals against experimental pneumonia challenge. Foals were immunized twice via gavage of R. equi (immunized group) or saline (control group) at ages 1 and 3 days. At age 28 days, all foals were challenged intrabronchially with R. equi. Post-challenge, all 5 immunized foals remained healthy, whereas 67% (4/6) of control foals developed clinical pneumonia. Immunized foals exhibit changes in the epigenetic profile of blood monocytes, > 1,000 differentially-expressed genes in neutrophils, higher concentrations of R. equi-specific IgG1 and IgG4/7, and a higher number of IFN-γ producing lymphocytes in response to R. equi stimulation indicating T helper type 1 response compared to control foals. Together, our data indicate that early life exposure to R. equi in the gastrointestinal tract can modulate innate immune responses, generate specific antibodies and cell-mediated immunity, and protect against pneumonia.
Keywords: Rhodococcus equi; Horse; Infection; Trained Immunity; Vaccine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

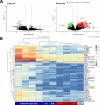
 = upregulated,
= upregulated,  = downregulated). (B) Heatmap of RNA-seq results of neutrophil from 28-day-old foals including all genes with fold-change higher than 11 between immunized and control groups (royal blue = healthy; red = pneumonic; gray = subclinical pneumonia).
= downregulated). (B) Heatmap of RNA-seq results of neutrophil from 28-day-old foals including all genes with fold-change higher than 11 between immunized and control groups (royal blue = healthy; red = pneumonic; gray = subclinical pneumonia).
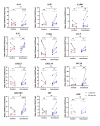
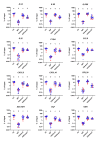

References
-
- Mohr, E. & Siegrist, C. A. Vaccination in early life: Standing up to the challenges. Curr. Opin. Immunol.41, 1–8. 10.1016/j.coi.2016.04.004 (2016). - PubMed
-
- Pargass, I. S. et al. The influence of age and Rhodococcus equi infection on CD1 expression by equine antigen presenting cells. Vet. Immunol. Immunopathol.130, 197–209. 10.1016/j.vetimm.2009.02.007 (2009). - PubMed
-
- Lopez, B. S. et al. The effect of age on foal monocyte-derived dendritic cell (MoDC) maturation and function after exposure to killed bacteria. Vet. Immunol. Immunopathol.210, 38–45. 10.1016/j.vetimm.2018.11.020 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

