Overwhelming glycyrrhizin production in Glycyrrhiza glabra induced by rihizobial symbiosis
- PMID: 40415147
- PMCID: PMC12228657
- DOI: 10.1007/s11418-025-01918-2
Overwhelming glycyrrhizin production in Glycyrrhiza glabra induced by rihizobial symbiosis
Abstract
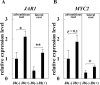
We reported that Glycyrrhiza uralensis inoculated with rhizobium tended to increase biomass production and glycyrrhizic acid (GL) production, in this study we have also achieved drastically increase in biomass and GL production in Glycyrrhiza glabra. At thirty days after inoculation (DAI), a significant increase in SPAD values was observed, and the expression of GL synthesis marker genes was also significantly increased. At 150 DAI, a significant increase in biomass was observed. Characteristically, it was also found that thick roots were enlarged by rhizobial inoculation. In addition, the expression of GL synthesis marker genes was also significantly increased. Moreover, GL content per unit root dry weight reached 4%, and GL production per plant increased six times compared to uninoculated plants. Moreover, we tried to reveal the mechanism of induction of GL production by rhizobial inoculation. Since it has been reported that the expression of jasmonic acid (JA) synthesis marker genes is increased by rhizobium in soybean, we investigated the expression of those genes in G. glabra, and found that GgMYC2 and GgJAR1 were up-regulated at Thirty DAI. Furthermore, methyl jasmonate treatment increased the expression of GL synthesis marker genes, suggesting that JA signaling is involved in the increased GL production due to rhizobial inoculation. These results aid in understanding the mechanism of increased GL production through the introduction of rhizobial symbiosis, and show the potential for providing a technology to significantly shorten the cultivation period for the production of Glycyrrhiza that meets the criteria for herbal medicines.
Keywords: Glycyrrhiza; Glycyrrhizic acid; Nodulation; Rhizobium; Symbiosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: All authors have and declare that: (i) no support, financial or otherwise, has been received from any organization that may have an interest in the submitted work; and (ii) there are no other relationships or activities that could appear to have influenced the submitted work.
Figures









References
-
- Han Y, Pang X, Zhang X, Han R, Liang Z (2022) Resource sustainability and challenges: status and competitiveness of international trade in licorice extracts under the Belt and Road Initiative. Global Ecol Conserv 34:e02014. 10.1016/j.gecco.2022.e02014 - DOI
-
- Ozaki K, Shibano M, Kusano G, Watanabe H (2010) Aim for production of Glycyrrhiza Radix in Japan (2) selection of pharmaceutically fine strains from Glycyrrhiza uralensis Fisher. Jpn Soc Pharmacogn 64:76–82
-
- The Society of Japanese Pharmacopoeia (2021) The Japanese Pharmacopoeia eighteenth edition. Yakuji-nippo
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

