Less frequent dosing of GLP-1 receptor agonists as a viable weight maintenance strategy
- PMID: 40415172
- PMCID: PMC12210098
- DOI: 10.1002/oby.24302
Less frequent dosing of GLP-1 receptor agonists as a viable weight maintenance strategy
Abstract
Objective: Incretin mimetics are revolutionizing obesity treatment, but high prices and supply shortages limit patient access. Some clinicians have suggested less frequent dosing as an off-ramping strategy to maintain weight loss, but this approach lacks published evidence regarding its weight loss efficacy. We aim to provide such clinical evidence and to rationalize these results with mathematical modeling.
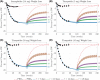
Methods: We present a real-world case series of two patients who took their incretin mimetic less frequently than recommended. We complement this case report with a pharmacokinetic-pharmacodynamic model of virtual patients that simulates long-term weight change with semaglutide and tirzepatide administered at various frequencies.
Results: Both real-world and virtual patients maintained significant weight loss under reduced dosing frequencies. Our results indicate that reducing frequency does not commensurately reduce efficacy. The majority of weight loss persists even when patients wait 2, 3, or perhaps even 4 weeks between doses.
Conclusions: Our findings support the hypothesis that less frequent administration of incretin mimetics can be a viable and cost-saving long-term weight maintenance strategy in conjunction with sustained lifestyle modification. Further research is warranted to validate the effectiveness of this off-label approach, define optimal dosing regimens to meet individual patient needs, and evaluate the cost-benefit implications.
© 2025 The Author(s). Obesity published by Wiley Periodicals LLC on behalf of The Obesity Society.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures
References
-
- Müllertz ALO, Sandsdal RM, Jensen SBK, Torekov SS. Potent incretin‐based therapy for obesity: A systematic review and meta‐analysis of the efficacy of semaglutide and tirzepatide on body weight and waist circumference, and safety. Obes Rev. 2024;25(5):e13717. - PubMed
-
- Gudzune KA, Kushner RF. Medications for obesity: A review. JAMA. 2024;332(7):571‐584. - PubMed
-
- Strathe A, Horn DB, Larsen MS, et al. A model‐based approach to predict individual weight loss with semaglutide in people with overweight or obesity. Diabetes Obes Metab. 2023;25(11):3171‐3180. - PubMed
-
- U.S. Food and Drug Administration . Clinical Pharmacology Review: NDA 215866. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/215866Orig1s000C...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

