Neurological and Cardiac Adverse Events in Cervical Cancer Treatment: A Case of Postoperative Sintilimab-Induced Encephalitis and Myocarditis
- PMID: 40415286
- PMCID: PMC12124153
- DOI: 10.12659/AJCR.947730
Neurological and Cardiac Adverse Events in Cervical Cancer Treatment: A Case of Postoperative Sintilimab-Induced Encephalitis and Myocarditis
Abstract
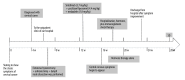
BACKGROUND Immune checkpoint inhibitors (ICIs) have shown considerable promise in enhancing patient outcomes and improving survival rates, offering a new frontier in cancer treatment. As a result, their use in clinical practice has become more widespread. However, the adverse effects associated with ICIs can compromise treatment efficacy. Among these, immune-related neurological adverse events are relatively uncommon, with an incidence rate of approximately 17%. Central nervous system (CNS) symptoms, although less frequent (around 6%), are particularly concerning due to their higher risk compared to peripheral nervous system involvement. Additionally, in recent years, the incidence of cardiac toxicity has been increasing, often indicated by elevated cardiac biomarkers, but most cases are asymptomatic. CASE REPORT This report presents a case of a middle-aged woman with cervical cancer who developed both encephalitis and myocarditis during postoperative consolidation therapy with an immune checkpoint inhibitor. A thorough evaluation, including laboratory tests, imaging studies, and an assessment of the patient's medical history and clinical presentation, excluded infection and paraneoplastic encephalitis as potential causes. She was treated with high-dose corticosteroids and intravenous immunoglobulin (IVIG), resulting in gradual resolution of her central nervous system symptoms and normalization of cardiac biomarkers. CONCLUSIONS Although the incidences of immune-related encephalitis and myocarditis are generally low, they can be very severe. With the increasing use of ICIs in clinical practice, the incidence of immune-related neurological symptoms may rise. This highlights the need for increased vigilance in clinical applications, including early preventive measures and prompt diagnosis and treatment to mitigate the adverse effects of these therapies, thereby maximizing patient benefits.
Conflict of interest statement
Figures




References
-
- Pang K, Shi ZD, Wei LY, et al. Research progress of therapeutic effects and drug resistance of immunotherapy based on PD-1/PD-L1 blockade. Drug Resist Updat. 2023;66:100907. - PubMed
-
- Chen Q, Wang C, Chen G, et al. Delivery strategies for immune checkpoint blockade. Adv Healthc Mater. 2018;7(20):e1800424. - PubMed
-
- Duong SL, Barbiero FJ, Nowak RJ, Baehring JM. Neurotoxicities associated with immune checkpoint inhibitor therapy. J Neurooncolo. 2021;152(2):265–77. - PubMed

