The Potential Use of Cannabidiol in the Treatment of Opioid Use Disorder: A Systematic Review
- PMID: 40415392
- PMCID: PMC12104536
- DOI: 10.1111/adb.70047
The Potential Use of Cannabidiol in the Treatment of Opioid Use Disorder: A Systematic Review
Abstract
Cannabidiol (CBD) has emerged as a potential treatment option for various psychiatric disorders, including substance use disorders. This systematic review is aimed at reviewing the evidence regarding the safety and efficacy of CBD as a therapeutic option in opioid use disorder (OUD) treatment in clinical and preclinical studies. We searched MEDLINE, Embase, PsycINFO, Scopus, Web of Science, CDSR and CENTRAL up to December 2023. We included original peer-reviewed human and animal studies evaluating CBD for OUD outcomes and excluded those that did not report OUD outcomes or used CBD solely with THC. The risk of bias was assessed with the Cochrane risk-of-bias tool for human studies and SYRCLE's tool for animal studies. Due to outcome heterogeneity, findings were presented using a qualitative synthesis. Four clinical studies (74 participants) and 16 preclinical studies met the inclusion criteria. The collective evidence from clinical and preclinical studies indicates that CBD holds promise as an adjunctive therapy for OUD with a well-tolerated profile during opioid use and withdrawal. Human clinical studies demonstrated a reduction in craving and alleviation of abstinence-induced anxiety. In preclinical studies, CBD has been shown to reduce withdrawal symptoms and diminish opioid-rewarding effects using the conditioned place preference paradigm, although the results are mixed, and not all preclinical studies reported these effects. The quality assessment for clinical studies indicated an overall evaluation of 'some concerns', while a notable level of 'unclear' risk was observed across the evaluated domains for preclinical studies. This systematic review highlights the potential of CBD as a beneficial treatment option for addressing cravings and anxiety symptoms during abstinence in individuals with OUD, based on findings from human studies. Continued research and clinical trials will be essential for further improving outcomes in OUD treatment using novel effective treatment approaches. Study limitations include the limited number of clinical studies, small sample size, short-term follow-up, lack of combination therapy and heterogeneity across preclinical studies. TRIAL REGISTRATION: PROSPERO identifier: CRD42023401446.
Keywords: addiction; cannabidiol; opioid; opioid use disorder; tetrahydrocannabinol; withdrawal.
© 2025 The Authors. Addiction Biology published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Conflict of interest statement
Dr. Bassir Nia is a member of the Scientific Advisory Board of Synendos Therapeutics AG, Switzerland. The other authors declare no conflicts of interest.
Figures


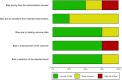

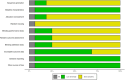
Similar articles
-
Adjunctive Management of Opioid Withdrawal with the Nonopioid Medication Cannabidiol.Cannabis Cannabinoid Res. 2022 Oct;7(5):569-581. doi: 10.1089/can.2021.0089. Epub 2021 Oct 22. Cannabis Cannabinoid Res. 2022. PMID: 34678050 Free PMC article. Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Use and perceptions of Cannabidiol among individuals in treatment for opioid use disorder.Harm Reduct J. 2024 Jul 17;21(1):135. doi: 10.1186/s12954-024-01051-5. Harm Reduct J. 2024. PMID: 39020418 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Cannabidiol Effect on Cue-Induced Craving for Individuals with Opioid Use Disorder Treated with Buprenorphine: A Small Proof-of-Concept Open-Label Study.Integr Med Rep. 2022 Aug 1;1(1):157-163. doi: 10.1089/imr.2022.0070. Epub 2022 Aug 26. Integr Med Rep. 2022. PMID: 36105269 Free PMC article.
References
-
- Shen J., Hua G., Li C., Liu S., Liu L., and Jiao J., “Prevalence, Incidence, Deaths, and Disability‐Adjusted Life‐Years of Drug Use Disorders for 204 Countries and Territories During the Past 30 Years,” Asian Journal of Psychiatry 86 (2023): 103677. - PubMed
-
- Carroll J. J., Green T. C., and Noonan R. K., Evidence‐Based Strategies for Preventing Opioid Overdose: What's Working in the United States: An Introduction for Public Heath, Law Enforcement, Local Organizations, and Others Striving to Serve Their Community, (2018).
-
- Mariolis T., Bosse J., Martin S., Wilson A., and Chiodo L., “A Systematic Review of the Effectiveness of Buprenorphine for Opioid Use Disorder Compared to Other Treatments: Implications for Research and Practice,” Journal of Addiction Research & Therapy 10, no. 2 (2019): 1000379.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

