MRI-based quantification of whole-organ renal metabolic rate of oxygen during free-breathing
- PMID: 40415411
- PMCID: PMC12309874
- DOI: 10.1002/mrm.30583
MRI-based quantification of whole-organ renal metabolic rate of oxygen during free-breathing
Abstract
Purpose: Renal metabolic rate of oxygen (rMRO2) reflects the kidney's metabolic efficiency, making it a potential biomarker for early-stage kidney disease. This study introduces an ungated, free-breathing MRI sequence in comparison to its breath-hold counterpart to noninvasively measure whole-organ rMRO2.
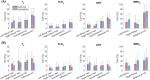
Methods: Free-breathing (FB) K-MOTIVE sequence (kidney metabolism of oxygen via T2 and interleaved velocity encoding) was developed to simultaneously measure renal blood flow rate (BFR) and T2 of blood water using the conservation of mass. T2 is converted to venous oxygen saturation (SvO2) using a calibration curve. Compared to previous versions, FB K-MOTIVE minimizes respiratory motion artifacts by acquiring fully sampled velocity maps with spiral readout instead of partially collecting radial views at each T2 weighting. Healthy participants (n = 15, 32 ± 9 years) were imaged at 3 T at the renal veins to quantify individual rMRO2, and at the suprarenal and infrarenal inferior vena cava to indirectly quantify bilateral rMRO2 (the total metabolism from both kidneys).
Results: Renal venous blood was highly oxygenated (SvO2 91% ± 3%) and exhibited high BFR of 460 ± 90 mL/min per kidney. Further, total rMRO2 of the two kidneys (160 ± 80 (μmol O2/min)/100 g) was statistically comparable to the indirect bilateral rMRO2 (250 ± 120 (μmol O2/min)/100 g, p = 0.066). Using Lin's concordance correlation coefficient, there was good agreement between breath-hold and free-breathing acquisitions at the individual kidneys for SvO2 (>0.75), BFR (>0.96), and rMRO2 (>0.75).
Conclusion: FB K-MOTIVE is a feasible approach to estimate rMRO2, yielding reproducible and physiologically plausible metabolic parameters. Free-breathing acquisition can enhance patient comfort by eliminating the need for breath-holding.
Keywords: MRI; kidney; metabolism; oxygenation.
© 2025 The Author(s). Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Figures
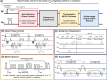
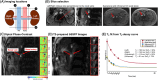
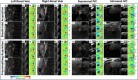
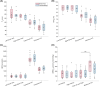


References
-
- Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867‐872. - PubMed
-
- Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end‐stage renal disease. Nat Rev Nephrol. 2010;6:667‐678. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

