Discovery of Pre-Clinical Candidate VU6008055/ AF98943: A Highly Selective, Orally Bioavailable, and Structurally Distinct Tricyclic M4 Muscarinic Acetylcholine Receptor Positive Allosteric Modulator (PAM) with Robust In Vivo Efficacy
- PMID: 40415588
- PMCID: PMC12142581
- DOI: 10.1021/acschemneuro.5c00277
Discovery of Pre-Clinical Candidate VU6008055/ AF98943: A Highly Selective, Orally Bioavailable, and Structurally Distinct Tricyclic M4 Muscarinic Acetylcholine Receptor Positive Allosteric Modulator (PAM) with Robust In Vivo Efficacy
Abstract
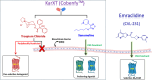
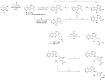
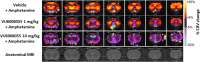
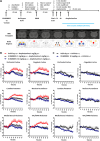
Herein, we report the structure-activity relationship to develop novel tricyclic M4 positive allosteric modulator scaffolds with improved pharmacological properties. This endeavor involved modifying a 5-amino-3,4-dimethylthieno[2,3-c]pyridazine-6-carboxamide core via a "tie-back" strategy to discover a novel tricyclic 3,4-dimethylpyrimido[4',5':4,5]thieno[2,3-c]pyridazine core. From this exercise, VU6008055/AF98943 was identified as a preclinical candidate, which displays low nanomolar potency against both human and rat M4. Moreover, VU6008055 is highly brain penetrant, has an overall superior pharmacological and DMPK profile to previously reported M4 PAMs, and demonstrates efficacy in preclinical models of antipsychotic-like activity.
Keywords: Alzheimer’s disease; M4; Parkinson’s disease; VU6008055; muscarinic acetylcholine receptor (mAChR); positive allosteric modulator (PAM); schizophrenia; structure–activity relationship (SAR).
Figures









References
-
- Shen W., Plotkin J. L., Francardo V., Ko W. K. D., Xie Z., Li Q., Fieblinger T., Wess J., Neubig R. R., Lindsley C. W., Conn P. J., Greengard P., Bezard E., Cenci M. A., Surmeier D. J.. M4 Muscarinic receptor signaling ameliorates striatal plasticity deficits in models of L-DOPA-induced dyskinesia. Neuron. 2015;88:762–773. doi: 10.1016/j.neuron.2015.10.039. - DOI - PMC - PubMed
-
- Pancani T., Foster D. J., Moehle M. S., Bichell T. J., Bradley E., Bridges T. M., Klar R., Poslusney M., Rook J. M., Daniels J. S., Niswender C. M., Jones C. K., Wood M. R., Bowman A. B., Lindsley C. W., Xiang Z., Conn P. J.. Allosteric activation of M4 muscarinic receptors improve behavioral and physiological alteration in early symptomatic YAC128 mice. Proc. Natl. Acad. Sci. U.S.A. 2015;112:14078–14083. doi: 10.1073/pnas.1512812112. - DOI - PMC - PubMed
-
- Foster D. J., Wilson J. M., Remke D. H., Mahmood M. S., Uddin M. J., Wess J., Patel S., Marnett L. J., Niswender C. M., Jones C. K., Xiang Z., Lindsley C. W., Rook J. M., Conn P. J.. Antipsychotic-like effects of M4 positive allosteric modulators are mediated by CB2 receptor-dependent inhibition of Dopamine release. Neuron. 2016;91:1244–1252. doi: 10.1016/j.neuron.2016.08.017. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

