Purity of Biosynthesized Eumelanin via Solid-State Nuclear Magnetic Resonance
- PMID: 40415792
- PMCID: PMC12096232
- DOI: 10.1021/acsomega.5c01034
Purity of Biosynthesized Eumelanin via Solid-State Nuclear Magnetic Resonance
Abstract
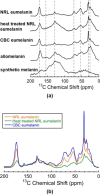
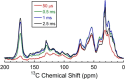
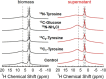
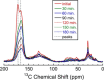
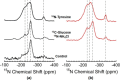
Eumelanins are a family of biomolecules with a wide range of functionality in nature and tremendous potential for commercial and industrial applications, provided that they can be produced in large quantities. Our group has developed a method to biosynthesize eumelanin with high yield using the marine bacterium Vibrio natriegens. In this work, we use high-resolution 1H, 13C, and 15N magic angle spinning (MAS) nuclear magnetic resonance (NMR) to characterize the purity of the extracted solid eumelanin as a function of the quality control methods. A key observation is that biomass eumelanin contains a higher level of biocontaminant compared to supernatant eumelanin. Additional insights into the efficiency of the biosynthetic pathway of eumelanin synthesis were obtained by incorporating 13C- and 15N-enriched precursors into the growth medium and subsequent MAS NMR spectra of the eumelanins. These results highlight the usefulness of solid-state NMR for assessing the quality of the eumelanins produced biosynthetically, which is critical for their further application.
© 2025 The Authors. Published by American Chemical Society.
Figures






References
-
- Cao W., Zhou X., McCallum N. C., Hu Z., Ni Q. Z., Kapoor U., Heil C. M., Cay K. S., Zand T., Mantanona A. J., Jayaraman A., Dhinojwala A., Deheyn D. D., Shawkey M. D., Burkart M. D., Rinehart J. D., Gianneschi N. C.. Unraveling the structure and function of eumelanin through synthesis. J. Am. Chem. Soc. 2021;143(7):2622–2637. doi: 10.1021/jacs.0c12322. - DOI - PubMed
-
- Wang Z., Tschirhart T., Schultzhaus Z., Kelly E. E., Chen A., Oh E., Nag O., Glaser E. R., Kim E., Lloyd P. F., Charles P. T., Li W., Leary D., Compton J., Phillips D. A., Dhinojwala A., Payne G. F., Vora G. J.. Eumelanin Produced by the Fast-Growing Marine Bacterium Vibrio natriegens through Heterologous Biosynthesis: Characterization and Application. Appl. Environ. Microbiol. 2020;86:e0274919. doi: 10.1128/AEM.02749-19. - DOI - PMC - PubMed
-
- d'Ischia M., Wakamatsu K., Cicoira F., Di Mauro E., Garcia-Borron J. C., Commo S., Galván I., Ghanem G., Kenzo K., Meredith P., Pezzella A., Santato C., Sarna T., Simon J. D., Zecca L., Zucca F. A., Napolitano A., Ito S.. Eumelanins and melanogenesis: from pigment cells to human health and technological application. Pigm. Cell Melanoma Res. 2015;28(5):520–544. doi: 10.1111/pcmr.12393. - DOI - PubMed
-
- Chatterjee S., Prados-Rosales R., Frases S., Itin B., Casadevall A., Stark R. E.. Using Solid-State NMR to Monitor the Molecular Consequences of Cryptococcus neoformans Melanization with Different Catecholamine Precursors. Biochemistry. 2012;51(31):6080–6088. doi: 10.1021/bi300325m. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
