Human beta defensin-2 protects the epithelial barrier during methicillin-resistant Staphylococcus aureus infection in chronic rhinosinusitis with nasal polyps
- PMID: 40415954
- PMCID: PMC12098561
- DOI: 10.3389/fcimb.2025.1551080
Human beta defensin-2 protects the epithelial barrier during methicillin-resistant Staphylococcus aureus infection in chronic rhinosinusitis with nasal polyps
Abstract
Objective: We investigated the effect of human beta defensin-2 (hBD-2) on nasal epithelial barrier function with methicillin-resistant Staphylococcus aureus (MRSA) infection in chronic rhinosinusitis with nasal polyps (CRSwNP).
Methods: The expression of hBD-2 was measured in nasal polyps (NPs) from CRSwNP. MRSA was treated with different concentrations of hBD-2 to assess the invasive ability. Primary human nasal epithelial cells (HNECs) cultured at the air-liquid interface (ALI) were pre-incubated with or without hBD-2 prior to MRSA infection. The cell viability, the epithelial cell integrity, and the tight junction (TJ) expression were evaluated.
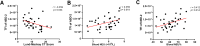
Results: The expression of hBD-2 in the CRSwNP group was higher than that in the control group. In addition, the hBD-2 protein was negatively correlated with the Lund-Mackay CT score and was positively correlated with the neutrophil levels in CRSwNP. The presence of hBD-2 significantly reduced the invasive ability of MRSA in HNECs. MRSA decreased the epithelial cell integrity by diminishing the protein expression of occludin and zonula occludens-1 (ZO-1). Furthermore, hBD-2 prevented the MRSA-induced barrier disruption by increasing the mucosal permeability and the expression of occludin and ZO-1.
Conclusion: The results suggest that hBD-2 may partially attenuate the epithelial barrier disruption induced by MRSA, indicating the protective effect of hBD-2 on S. aureus infection.
Keywords: chronic rhinosinusitis with nasal polyps; epithelial barrier; human beta defensin-2; methicillin-resistant Staphylococcus aureus; tight junctions.
Copyright © 2025 Tian, Hsu, Sun, Shi, Hu, Wu, Zhao and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

