Primary cell culture systems to investigate host-pathogen interactions in bacterial respiratory tract infections of livestock
- PMID: 40415959
- PMCID: PMC12098631
- DOI: 10.3389/fcimb.2025.1565513
Primary cell culture systems to investigate host-pathogen interactions in bacterial respiratory tract infections of livestock
Abstract
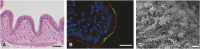
Respiratory infections of livestock represent a major health issue for the animals and cause high economic losses for the farmers. Still, little is known about the intricate interactions between host cells and the many different pathogens that cause respiratory diseases, leaving a substantial knowledge gap to be filled in order to develop effective therapies. Immortalized cell lines and two-dimensional cultures of primary respiratory epithelial cells do not reflect the complex architecture and functionality of the respiratory tract tissues. Thus, it is essential to develop and apply appropriate primary cell culture systems to study respiratory diseases. In human research, the use of complex cell culture systems, such as air-liquid interface (ALI) cultures, organoids and lung-on-chip, has proceeded significantly during the last years, whereas in veterinary research, these models are only rarely used. Nevertheless, there are several three-dimensional, primary cell culture systems available to study respiratory infections of livestock. Here, we give an overview on models that are currently used in this field: nasal mucosa explants, tracheal organ cultures, ALI cultures, and precision-cut lung slices. All these models align with the 3R principle, as they can replace animal experiments to some extent and the tissue material for these culture systems can be obtained from abattoirs or veterinary research facilities. We aim to encourage other researchers to use these versatile cell culture systems to drive investigations of respiratory tract infections of livestock forward. Finally, these models are not limited to infection research, but can also be applied in other research fields and can be transferred to other animal species than livestock.
Keywords: air-liquid interface cultures; livestock diseases; nasal mucosa explants; organotypic models; precision-cut lung slices; respiratory tract infections; tracheal organ cultures.
Copyright © 2025 Weldearegay, Brogaard, Rautenschlein, Meens, Valentin-Weigand and Schaaf.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Abraham G., Zizzadoro C., Kacza J., Ellenberger C., Abs V., Franke J., et al. . (2011). Growth and differentiation of primary and passaged equine bronchial epithelial cells under conventional and air-liquid-interface culture conditions. BMC Vet Res. 7, 26. doi: 10.1186/1746-6148-7-26 - DOI - PMC - PubMed
-
- Abugisisa L., Royse E. X., Kemp M. W., Jobe A. H., Hillman N. H. (2023). Preterm ovine respiratory epithelial cell responses to mechanical ventilation, lipopolysaccharide, and interleukin-13. Am. J. Physiol. Lung Cell Mol. Physiol. 324, L815–L824. doi: 10.1152/ajplung.00355.2022 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

