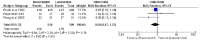Racecadotril Versus Loperamide for Acute Diarrhea of Infectious Origin in Adults: A Systematic Review and Meta-Analysis
- PMID: 40415979
- PMCID: PMC12098968
- DOI: 10.1002/hsr2.70849
Racecadotril Versus Loperamide for Acute Diarrhea of Infectious Origin in Adults: A Systematic Review and Meta-Analysis
Abstract
Background and aims: Loperamide and racecadotril are two antidiarrheal medications with different mechanisms of action that are highly important for the treatment of diarrhea as a result of infectious pathology. Acute infectious diarrhea has a profound impact on our surroundings because of its detrimental effects on individual health. Medication such as racecadotril, among various other drug classes, plays a pivotal role in treating these diseases via the management of symptoms through its antisecretory, proabsorptive effects on the intestinal tract. The main objective of this analysis was to evaluate and contrast the usefulness and safety profiles of these drugs by combining information from randomized controlled trials and highlighting important side effects reported, such as constipation and stomach discomfort.
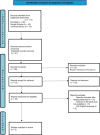
Method: A total of 117 records were found after a thorough literature search was carried out across several databases, including PubMed, Google Scholar, and the Cochrane Library. The Cochrane Collaboration technique was utilized to evaluate the potential for bias. RevMan 5.2 data analysis was performed via a random effects model. The results are displayed as the mean difference (MD) with 95% CI for continuous data and relative risk (RR) with 95% confidence intervals (CIs) for dichotomous data. Statistical significance was established at p < 0.05.
Results: Compared with loperamide, racecadotril significantly improved the clinical response (relative risk [RR] and 95% confidence interval [CI]). Additionally, the analysis of secondary outcomes revealed varying effects on abdominal pain, constipation, and abdominal enlargement, with moderate heterogeneity observed (I² = 56%).
Conclusion: Compared with loperamide, racecadotril is a better therapeutic option for adult diarrhea caused by infection.
Keywords: diarrhea; infectious origin; loperamide; meta‐analysis; racecadotril; randomized controlled trials.
© 2025 The Author(s). Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
A blind, randomized comparison of racecadotril and loperamide for stopping acute diarrhea in adults.World J Gastroenterol. 2005 Mar 14;11(10):1540-3. doi: 10.3748/wjg.v11.i10.1540. World J Gastroenterol. 2005. PMID: 15770734 Free PMC article. Clinical Trial.
-
Racecadotril for acute diarrhoea in children.Cochrane Database Syst Rev. 2019 Dec 19;12(12):CD009359. doi: 10.1002/14651858.CD009359.pub2. Cochrane Database Syst Rev. 2019. PMID: 31858591 Free PMC article.
-
A multinational comparison of racecadotril and loperamide in the treatment of acute watery diarrhoea in adults.Scand J Gastroenterol. 2002 Jun;37(6):656-61. doi: 10.1080/00365520212495. Scand J Gastroenterol. 2002. PMID: 12126242 Clinical Trial.
-
A Comprehensive Comparison of the Efficacy and Tolerability of Racecadotril with Other Treatments of Acute Diarrhea in Adults.Front Med (Lausanne). 2016 Oct 14;3:44. doi: 10.3389/fmed.2016.00044. eCollection 2016. Front Med (Lausanne). 2016. PMID: 27790616 Free PMC article. Review.
-
Racecadotril Versus Loperamide in Acute Radiation Enteritis: A Randomized, Double-Masked, Phase 3, Noninferiority Trial.Int J Radiat Oncol Biol Phys. 2024 Mar 1;118(3):616-625. doi: 10.1016/j.ijrobp.2023.09.021. Epub 2023 Sep 22. Int J Radiat Oncol Biol Phys. 2024. PMID: 37742773 Clinical Trial.
References
-
- World Health Organization , “Diarrheal Disease,” World Health Organization, published 2024, https://www.who.int/en/news-room/fact-sheets/detail/diarrhoeal-disease.
-
- E. S. Meisenheimer, Epstein C. D., and D. Thiel, “Acute Diarrhea in Adults,” American Family Physician 106, no. 1 (2022): 72–80. - PubMed
-
- Siciliano V., Nista E. C., Rosà T., Brigida M., and Franceschi F., “Clinical Management of Infectious Diarrhea,” Reviews on Recent Clinical Trials 15, no. 4 (2021): 298–308. - PubMed
-
- “Definition & Facts for Diarrhea,” National Institute of Diabetes and Digestive and Kidney Diseases, https://www.niddk.nih.gov/health-information/digestive-diseases/diarrhea....
-
- Maharani B., “Screening Methods for the Evaluation of Antidiarrheal Drugs.” In Introduction to Basics of Pharmacology and Toxicology: Volume 3: Experimental Pharmacology: Research Methodology and Biostatistics, eds. Lakshmanan M., Shewade D. G., and Raj G. M. (Springer Nature Singapore, 2022), 383–389.
Publication types
LinkOut - more resources
Full Text Sources