Exploring substitution effects on the potential dominant conformations of NBF derivatives leading to functional conversion at the mu opioid receptor
- PMID: 40416448
- PMCID: PMC12096176
- DOI: 10.1039/d5cb00036j
Exploring substitution effects on the potential dominant conformations of NBF derivatives leading to functional conversion at the mu opioid receptor
Abstract
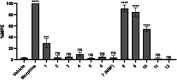
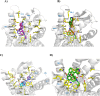
We previously identified NBF (β-configuration at C6) and its 6α-counterpart as mu opioid receptor (MOR) antagonists. To explore the effect of C6 conformation of the epoxymorphinan ring on their MOR function, five pairs of NBF derivatives bearing both 6α and 6β configurations with substitutions on the 3'-position of the benzofuran ring were synthesized. In vitro and in vivo studies demonstrated that compounds carrying phenyl and 4-pyridine substituents retained their antagonistic properties independent of the C6 configuration. Halogen and methyl substituents with the 6α-configuration remained as MOR antagonists, while their 6β-counterparts switched to MOR agonists. Molecular modeling studies indicated that the C6 configuration and structural modification may collectively decide the orientation of the benzofuran ring, leading to conformation retention or a switch within the MOR binding pocket. These results together aid the understanding of the NBF structure-activity relationship (SAR) and provide insights for functional conversion at the MOR, supporting future endeavors to develop novel MOR ligands.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



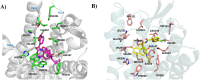
Similar articles
-
SAR Matrices Enable Discovery of Mixed Efficacy μ-Opioid Receptor Agonist Peptidomimetics with Simplified Structures through an Aromatic-Amine Pharmacophore.ACS Chem Neurosci. 2021 Jan 6;12(1):216-233. doi: 10.1021/acschemneuro.0c00693. Epub 2020 Dec 21. ACS Chem Neurosci. 2021. PMID: 33346631 Free PMC article.
-
Mu-opioid antagonists for opioid-induced bowel dysfunction in people with cancer and people receiving palliative care.Cochrane Database Syst Rev. 2018 Jun 5;6(6):CD006332. doi: 10.1002/14651858.CD006332.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2022 Sep 15;9:CD006332. doi: 10.1002/14651858.CD006332.pub4. PMID: 29869799 Free PMC article. Updated.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Opioids for cancer pain - an overview of Cochrane reviews.Cochrane Database Syst Rev. 2017 Jul 6;7(7):CD012592. doi: 10.1002/14651858.CD012592.pub2. Cochrane Database Syst Rev. 2017. PMID: 28683172 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

