Magnetic carbon nanotube-catalyzed multicomponent synthesis of 5-substituted-1 H-tetrazoles
- PMID: 40416636
- PMCID: PMC12100474
- DOI: 10.1039/d5ra02641e
Magnetic carbon nanotube-catalyzed multicomponent synthesis of 5-substituted-1 H-tetrazoles
Abstract
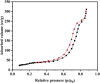
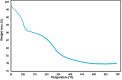
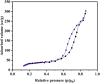
This study presents a pioneering approach with the introduction of a novel magnetic carbon nanotube composite, AlFe2O4-MWCNT-TEA-Ni(ii), designed as an efficient catalyst for the multicomponent synthesis of 5-substituted-1H-tetrazoles (18 examples, yields 89-98%). The primary objectives were to develop and evaluate this catalyst's ability to promote the formation of tetrazoles under eco-friendly conditions. The methods involved synthesizing the catalyst by combining AlFe2O4, MWCNTs, TEA, and Ni(ii), then testing its catalytic activity using various aromatic aldehydes, hydroxylamine, and sodium azide in DMF at 50 °C. The reaction parameters, including temperature, catalyst amount, and reaction time, were optimized for maximum yield and selectivity. Results showed that the catalyst achieved high yields of tetrazoles and demonstrated significant selectivity in a remarkably short reaction time, showcasing its exceptional efficiency. The magnetism of AlFe2O4 facilitated easy recovery and recyclability (seven runs), underscoring the catalyst's sustainability. This system's advantages include its high activity, reusability, and environmentally friendly nature, making it a promising approach for green nitrogen-rich heterocycle synthesis. Prospects involve exploring the catalyst's potential in large-scale applications, expanding its use to other heterocyclic syntheses, and investigating its performance with different substrates. This work underscores the significant role of integrating magnetic nanomaterials into catalytic systems to promote sustainable and efficient organic synthesis methods, offering a promising future for the field.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Jin J. Xu R. Wu X. Fang X. Kong W. Zhang K. Cheng J. Tetrahedron. 2023;132:133261.
-
- Zarezin D. P. Kabylda A. M. Vinogradova V. I. Dorovatovskii P. V. Khrustalev V. N. Nenajdenko V. G. Tetrahedron. 2018;74:4315–4332.
-
- Javahershenas R. Mei H. Koleyc M. Soloshonokd V. A. Makarem A. Synthesis. 2024;56:2445–2461.
-
- Jaiswal S. Verma K. Dwivedi J. Sharma S. Eur. J. Med. Chem. 2024;271:116388. - PubMed
LinkOut - more resources
Full Text Sources

