Case Report: Acral vasculitis induced by Immune Checkpoint Inhibitors: a case series and literature review
- PMID: 40416866
- PMCID: PMC12098104
- DOI: 10.3389/fonc.2025.1537825
Case Report: Acral vasculitis induced by Immune Checkpoint Inhibitors: a case series and literature review
Abstract
Introduction: Immune Checkpoint Inhibitors (ICIs) may cause various immune- related Adverse Events (irAEs). Of these events, vascular involvement is still considered an uncommon irAEs and generally concerns large or medium vessels. Acral small-vessel vasculitis can lead to severe digital necrosis.
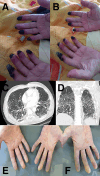
Case presentation: Herein, we present three cases after treatment with pembrolizumab, nivolumab, and combination nivolumab/ipilimumab for lung adenocarcinoma, renal cell carcinoma, and melanoma, respectively. Two patients had a Raynaud's-like syndrome. All of them presented with digital ischemia of both hands and with severe acral necrosis in the first case. Management consisted in ICI discontinuation, high-dose steroids, and vasodilator agents with good evolution in the three cases. No rechallenge of ICI has been attempted.
Discussion: We found 12 other cases in the literature review to build a cohort of 15 patients, mostly male with a median age of 60 years. Lung cancer and melanoma are the most common tumors. The most frequently used ICI was pembrolizumab. The median time to onset was 8 weeks. The main clinical presentation was a distal and painful necrosis mostly on thehands with bilateral involvement. Toes were affected in only two cases. All cases were severe features with grade ≥ 3. Eleven patients were treated with steroids and vasodilator agents. ICI was discontinued permanently in all patients.
Conclusion: ICI-induced small-vessel vasculitis can lead to severe digital ischemia, often in males, and is preceded by a Raynaud's-like syndrome with mostly bilateral and hand involvement. Data are still missing to optimize management of these kinds of patients.
Keywords: acral vasculitis; immune checkpoint inhibitors; immune-related adverse events; ipilimumab; nivolumab; pembrolizumab.
Copyright © 2025 Rivet, Guillon, Sibaud, Dion, Pastissier, Delavigne, Cougoul, Rauzy and Comont.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Le Burel S, Champiat S, Mateus C, Marabelle A, Michot JM, Robert C, et al. . Prevalence of immune-related systemic adverse events in patients treated with anti-Programmed cell Death 1/anti-Programmed cell Death-Ligand 1 agents: A single-centre pharmacovigilance database analysis. Eur J Cancer. (2017) 82:34–44. doi: 10.1016/j.ejca.2017.05.032 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources