Engineering safe anti-CD19-CD28ζ CAR T cells with CD8a hinge domain in serum-free media for adoptive immunotherapy
- PMID: 40416968
- PMCID: PMC12098533
- DOI: 10.3389/fimmu.2025.1545549
Engineering safe anti-CD19-CD28ζ CAR T cells with CD8a hinge domain in serum-free media for adoptive immunotherapy
Abstract
Background: Despite the curative potential, high cost of manufacturing and the toxicities limits the wider access of Chimeric Antigen Receptor (CAR) T cell therapy in global medicine. CARs are modular synthetic antigen receptors integrating the single-chain variable fragment (scFv) of an immunoglobulin molecule to the TCR signaling. CARs allow HLA independent, T cell mediated destruction of tumor cells independent of tumor associated-HLA downregulation and survive within the patient as 'living drug.' Here we report a safer approach for engineering alpha beta T cells with anti- CD19-CD28ζ CAR using self-inactivating (SIN) lentiviral vectors for adoptive immunotherapy.
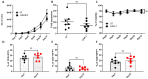
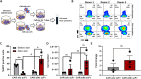
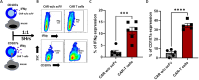
Method: αβ T cells from the peripheral blood (PB) were lentivirally transduced with CAR construct containing hinge domain from CD8α, transmembrane and co-stimulatory domain from CD28 along with signaling domain from CD3ζ and driven by human UBC promoter. The cells were pre-stimulated through CD3/CD28 beads before lentiviral transduction. Transduction efficiency, fold expansion and phenotype were monitored for the CAR T cells expanded for 10-12 days. The antigen-specific tumor-killing capacity of CD19 CAR T cells was assessed against a standard CD19 expressing NALM6 cell lines with a flow cytometry-based assay optimized in the lab.
Results and conclusion: We have generated high titer lentiviral vectors of CAR with a titer of 9.85 ± 2.2×107 TU/ml (mean ± SEM; n=9) generating a transduction efficiency of 27.57 ± 2.4%. (n=7) at an MOI of 10 in total T cells. The product got higher CD8+ to CD4+ CAR T cell ratio with preponderance of an effector memory phenotype on day 07 and day 12. The CAR-T cells expanded (148.4 ± 29 fold; n=7) in serum free media with very high viability (87.8 ± 2.2%; n=7) on day 12. The antitumor functions of CD19 CAR T cells as gauged against percentage lysis of NALM6 cells at a 1:1 ratio is 27.68 ± 6.87% drawing up to the release criteria. CAR T cells produced IFNγ (11.23 ± 1.5%; n=6) and degranulation marker CD107α (34.82 ± 2.08%; n=5) in an antigen-specific manner. Furthermore, the sequences of WPRE, GFP, and P2A were removed from the CAR construct to enhance safety. These CAR T cells expanded up to 21.7 ± 5.53 fold with 82.7±5.43% viability (n=4).
Conclusion: We have generated, validated, and characterized a reproducible indigenous workflow for generating anti-CD19 CAR T cells in vitro. This approach can be used for targeting cancer and autoimmune diseases in which CD19+ B lineage cells cause host damage.
Keywords: B lineage malignancies; CD19 CAR T cells; NALM-6; SIN vector; serum free media.
Copyright © 2025 Muthuvel, Ganapathy, Spencer, Raikar, Thangavel, Srivastava and Martin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains.Mol Ther. 2017 Nov 1;25(11):2452-2465. doi: 10.1016/j.ymthe.2017.07.013. Epub 2017 Jul 27. Mol Ther. 2017. PMID: 28807568 Free PMC article.
-
In vitro functional validation of anti-CD19 chimeric antigen receptor T cells expressing lysine-specific demethylase 1 short hairpin RNA for the treatment of diffuse large B cell lymphoma.Front Immunol. 2025 Jan 13;15:1521778. doi: 10.3389/fimmu.2024.1521778. eCollection 2024. Front Immunol. 2025. PMID: 39872520 Free PMC article.
-
A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines.J Immunother Cancer. 2017 May 16;5:42. doi: 10.1186/s40425-017-0246-1. eCollection 2017. J Immunother Cancer. 2017. PMID: 28515942 Free PMC article.
-
Recent advances in CAR-T cell engineering.J Hematol Oncol. 2020 Jul 2;13(1):86. doi: 10.1186/s13045-020-00910-5. J Hematol Oncol. 2020. PMID: 32616000 Free PMC article. Review.
-
CD19 CAR T cells for B cell malignancies: a systematic review and meta-analysis focused on clinical impacts of CAR structural domains, manufacturing conditions, cellular product, doses, patient's age, and tumor types.BMC Cancer. 2024 Aug 22;24(1):1037. doi: 10.1186/s12885-024-12651-6. BMC Cancer. 2024. PMID: 39174908 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

