Mechanisms of the FLASH effect: current insights and advances
- PMID: 40417178
- PMCID: PMC12098440
- DOI: 10.3389/fcell.2025.1575678
Mechanisms of the FLASH effect: current insights and advances
Abstract
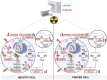
Radiotherapy is a fundamental tool in cancer treatment, utilized in over 60% of cancer patients during their treatment course. While conventional radiotherapy is effective, it has limitations, including prolonged treatment durations, which extend patient discomfort, and toxicity to surrounding healthy tissues. FLASH radiotherapy (FLASH-RT), an innovative approach using ultra-high-dose-rate irradiation, has shown potential in selectively sparing normal tissues while maintaining unaltered tumor control. However, the precise mechanisms underlying this "FLASH effect" remain unclear. This mini-review explores key hypotheses, including oxygen depletion, radical-radical interactions, mitochondrial preservation, differential DNA damage repair, and immune modulation. Oxygen levels significantly affect tissue response to radiation by promoting radical recombination, preserving mitochondrial function, and differentially activating DNA repair pathways in normal versus tumor tissues. However, the extent to which oxygen depletion contributes to the FLASH effect remains debated. Additionally, FLASH-RT may modulate the immune response, reducing inflammation and preserving immune cell function. To further enhance its therapeutic potential, FLASH-RT is increasingly being combined with complementary strategies such as radioprotectors, immunomodulators, and nanotechnology platforms. These combinations aim to amplify tumor control while further reducing normal tissue toxicity, potentially overcoming current limitations. Despite promising preclinical evidence, the exact mechanisms and clinical applicability of FLASH-RT require further investigation. Addressing these gaps is crucial for optimizing FLASH-RT and translating its potential into improved therapeutic outcomes for cancer patients. Continued research is essential to harness the full benefits of the FLASH effect, offering a paradigm shift in radiation oncology.
Keywords: cancer cells; cancer metabolism; cell death; flash; radiotherapy; ultra-high-dose rate irradiation.
Copyright © 2025 Rosini, Ciarrocchi and D’Orsi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Almeida A., Godfroid C., Leavitt R. J., Montay-Gruel P., Petit B., Romero J., et al. (2024b). Antitumor effect by either FLASH or conventional dose rate irradiation involves equivalent immune responses. Int. J. Radiat. Oncol. Biol. Phys. 118 (4), 1110–1122. 10.1016/j.ijrobp.2023.10.031 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

