Combining transcranial direct current stimulation with music therapy improves cognitive function in schizophrenia: study protocol for a randomized, double-blind, sham-controlled clinical trial
- PMID: 40417274
- PMCID: PMC12100747
- DOI: 10.3389/fpsyt.2025.1543789
Combining transcranial direct current stimulation with music therapy improves cognitive function in schizophrenia: study protocol for a randomized, double-blind, sham-controlled clinical trial
Abstract
Background: Despite numerous pharmacological treatments, individuals with schizophrenia continue to exhibit significant residual cognitive impairments, adversely affecting the progression of the illness and their overall quality of life. Preliminary evidence indicates that transcranial direct current stimulation (tDCS) and music therapy (MT) may offer potential benefits for enhancing cognitive function in schizophrenia. This study aims to examine the synergistic efficacy of tDCS and MT on cognitive impairments in individuals with schizophrenia and to elucidate the potential mechanisms involved in this process.
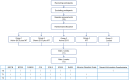
Methods: The study is designed as a randomized, double-blind, sham-controlled trial. All patients with schizophrenia will be randomly assigned to one of five groups: active tDCS combined with MT group, sham tDCS combined with MT group, active tDCS group, MT group, and a control group. The anodal electrode of tDCS will be positioned over the medial prefrontal cortex (mPFC), while the cathodal electrode will be placed over the visual cortex. MT will incorporate both Western Mozart and traditional Chinese classical music. The protocol involves 30-minute sessions conducted once daily, 5 days per week, for 4 consecutive weeks. The primary outcome measure is change in cognitive function, secondary outcomes include changes in psychotic symptoms, social function, and quality of life. Assessments will be evaluated at baseline (T0), after 2 weeks (T1), and after 4 weeks (T2). Furthermore, we will employ functional near-infrared spectroscopy (fNIRS) to examine hemodynamic changes on the cerebral cortex, and explore the neural effects of this combined treatment approach.
Discussion: This study proposes an innovative non-pharmacological treatment protocol that combines tDCS targeting the mPFC with MT to improve cognitive impairments in schizophrenia. As a proof-of-concept study, it aims to provide empirical evidence for the effectiveness of this combined intervention. Moreover, this study seeks to elucidate the underlying neural mechanisms and offer a rigorous framework for future clinical trials, ultimately providing a novel therapeutic strategy for enhancing cognitive functions in patients with schizophrenia.
Clinical trial registration: https://www.chictr.org.cn/, identifier, ChiCTR2400093161.
Trial registration details: The study is registered with https://www.chictr.org.cn/ under protocol registration number ChiCTR2400093161 (date of registration: 29. November. 2024). It was approved by the Research Ethics Committee of the Second Affiliated Hospital of Xinxiang Medical University (Approval Code: XYEFYLL-2024-82, Approval Date: 6 November 2024). Recruitment began in December 2024.
Keywords: cognitive functions; music therapy; schizophrenia; study protocol; transcranial direct current stimulation.
Copyright © 2025 Wei, He, Luo, Su, Chen, Qin, Zhang, Liu, Wei, Wang, Wang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The effect of transcranial direct current stimulation (tDCS) on cognitive function recovery in patients with depression following electroconvulsive therapy (ECT): protocol for a randomized controlled trial.BMC Psychiatry. 2024 Feb 16;24(1):130. doi: 10.1186/s12888-024-05567-9. BMC Psychiatry. 2024. PMID: 38365634 Free PMC article.
-
Optimizing Rehabilitation for Phantom Limb Pain Using Mirror Therapy and Transcranial Direct Current Stimulation: A Randomized, Double-Blind Clinical Trial Study Protocol.JMIR Res Protoc. 2016 Jul 6;5(3):e138. doi: 10.2196/resprot.5645. JMIR Res Protoc. 2016. PMID: 27383993 Free PMC article.
-
The effect of transcranial direct current stimulation combined with working memory training on working memory deficits in schizophrenic patients: study protocol for a randomized controlled trial.Trials. 2022 Sep 30;23(1):826. doi: 10.1186/s13063-022-06776-x. Trials. 2022. PMID: 36175919 Free PMC article.
-
Efficacy and auditory biomarker analysis of fronto-temporal transcranial direct current stimulation (tDCS) in targeting cognitive impairment associated with recent-onset schizophrenia: study protocol for a multicenter randomized double-blind sham-controlled trial.Trials. 2023 Feb 24;24(1):141. doi: 10.1186/s13063-023-07160-z. Trials. 2023. PMID: 36829240 Free PMC article.
-
Study protocol of a double-blind randomized control trial of transcranial direct current stimulation in post-stroke fatigue.Front Neurol. 2024 Jan 29;14:1297429. doi: 10.3389/fneur.2023.1297429. eCollection 2023. Front Neurol. 2024. PMID: 38348114 Free PMC article.
Cited by
-
Adjunctive transcranial direct current stimulation for cognitive improvement in schizophrenia: insights from a systematic review and exploratory meta-analysis.Front Psychiatry. 2025 Jul 11;16:1617068. doi: 10.3389/fpsyt.2025.1617068. eCollection 2025. Front Psychiatry. 2025. PMID: 40717779 Free PMC article.
-
Efficacy and mechanisms underlying MRI-guided high-definition transcranial direct current stimulation for depression among university students: study protocol for a stepped wedge cluster randomized controlled trial.BMC Psychiatry. 2025 Jul 1;25(1):630. doi: 10.1186/s12888-025-07118-2. BMC Psychiatry. 2025. PMID: 40597967 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

