Evaluating the Role of the Renin-angiotensin System in COVID-19: Implications for ACE Inhibitor and ARB Use During SARS-CoV-2 Infection
- PMID: 40417281
- PMCID: PMC12101613
- DOI: 10.33696/immunology.6.213
Evaluating the Role of the Renin-angiotensin System in COVID-19: Implications for ACE Inhibitor and ARB Use During SARS-CoV-2 Infection
Abstract
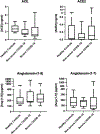
This study aimed to investigate the role of the renin-angiotensin system (RAS) in COVID-19, particularly focusing on key components such as ACE, ACE2, and their related peptides, angiotensin-(1-7) and angiotensin-(1-9). Using serum samples from healthy controls and both non-severe and severe COVID-19 patients, ELISA assays revealed no significant differences in these RAS components between the groups. In addition, in vitro studies showed no impact of ACE inhibitors or Angiotensin Receptor Blockers (ARB) on cell viability during SARS-CoV-2 infection. These clinical findings suggest that RAS alterations may not be a major factor in COVID-19 severity and the in vitro data support current guidelines, indicating the safety of continuing ACE inhibitors and ARBs in COVID-19 patients without evidence of increased SARS-CoV-2 infectivity in the presence of these compounds. This study highlights the lack of significant changes in key RAS components during COVID-19 in a clinical cohort and provides critical in vitro evidence supporting the continued use of ACE inhibitors and ARBs in treating patients.
Keywords: COVID-19; Cardiovascular disease; Coronavirus disease; Diabetes; Hypertension.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Figures
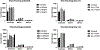
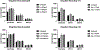
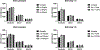
Similar articles
-
SARS-CoV-2 and the Angiotensin-Converting Enzyme 2 Receptor: Angiotensin-Converting Enzyme Inhibitor/Angiotensin 2 Receptor Blocker Utilization and a Shift Towards the Renin-Angiotensin-Aldosterone System Classical Pathway.Cureus. 2024 Mar 5;16(3):e55563. doi: 10.7759/cureus.55563. eCollection 2024 Mar. Cureus. 2024. PMID: 38576704 Free PMC article. Review.
-
[Effect of components of the renin-angiotensin system, rs2106809 polymorphism of the ACE2 gene, and therapy with RAS blockers on the severity of COVID-19].Probl Endokrinol (Mosk). 2023 Aug 30;69(4):21-31. doi: 10.14341/probl13274. Probl Endokrinol (Mosk). 2023. PMID: 37694864 Free PMC article. Russian.
-
Effects of renin-angiotensin system inhibition on end-organ protection: can we do better?Clin Ther. 2007 Sep;29(9):1803-24. doi: 10.1016/j.clinthera.2007.09.019. Clin Ther. 2007. PMID: 18035185 Review.
-
Renin-Angiotensin System Inhibitors and COVID-19: a Systematic Review and Meta-Analysis. Evidence for Significant Geographical Disparities.Curr Hypertens Rep. 2020 Sep 10;22(11):90. doi: 10.1007/s11906-020-01101-w. Curr Hypertens Rep. 2020. PMID: 32910274 Free PMC article.
-
Therapy with RAS inhibitors during the COVID-19 pandemic.J Cardiovasc Med (Hagerstown). 2021 May 1;22(5):329-334. doi: 10.2459/JCM.0000000000001160. J Cardiovasc Med (Hagerstown). 2021. PMID: 33795584 Review.
References
-
- Bavishi C, Maddox TM, Messerli FH. Coronavirus Disease 2019 (COVID-19) Infection and Renin Angiotensin System Blockers. JAMA Cardiol. 2020. Jul 1;5(7):745–7. - PubMed
-
- Smith DK, Lennon RP, Carlsgaard PB. Managing Hypertension Using Combination Therapy. Am Fam Physician. 2020. Mar 15;101(6):341–9. - PubMed
-
- Jia S, Liu Y, Yuan J. Evidence in Guidelines for Treatment of Coronary Artery Disease. Adv Exp Med Biol. 2020;1177:37–73. - PubMed
-
- Lemley KV. When to initiate ACEI/ARB therapy in patients with type 1 and 2 diabetes. Pediatr Nephrol. 2010. Oct;25(10):2021–34. - PubMed
-
- Azad GN, Kumar A. ACEi/ARB and Deaths of COVID-19 Patients. Curr Hypertens Rev. 2022;18(2):158–62. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
