Ultrafast solvent migration in an iron complex revealed by nonadiabatic dynamics simulations
- PMID: 40417306
- PMCID: PMC12100520
- DOI: 10.1039/d5sc01174d
Ultrafast solvent migration in an iron complex revealed by nonadiabatic dynamics simulations
Abstract
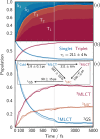
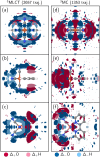
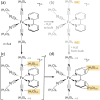
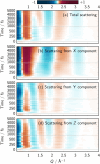
The response of a solvation shell to molecular solute photoexcitation is an ubiquitous phenomenon of great relevance in chemistry. This response can occur within just few tens of femtoseconds, making it very challenging to resolve experimentally. Thus, the details of the (an)isotropy of the solvent response around a solute, the presence of coherent solvent fluctuations, hydrogen bond reorganization mechanisms, and the intricate interplay between electronic, spin, nuclear, and solvent dynamics remain elusive. Here, we report large-scale nonadiabatic molecular dynamics simulations of [Fe(CN)4(bipy)]2- (bipy=2,2'-bipyridine) in water, where the electronic evolution from singlet metal-to-ligand charge transfer (MLCT) states to triplet MLCT and metal-centered (MC) states overlaps temporally with the molecule's nuclear motion and a strong solvent shell response. We leverage vibronic coupling model potentials combined with electrostatic embedding, within our so-called vibronic coupling/molecular mechanics (VC/MM) method, to be able to compute several thousand nonadiabatic excited-state trajectories, including all relevant singlet and triplet states as well as several thousand explicit water molecules. This superior statistics affords an unprecedented view on the three-dimensional solvent distribution dynamics at few-fs and sub-Å resolution. The results reveal a direct solvent migration mechanism, where excitation to the MLCT states leads to the breaking of hydrogen bonds to the cyanide ligands within less than 100 fs, followed by the formation of hydrogen bonds with the negatively charged bipyridyl ligand by the same water molecules. Furthermore, the MLCT and MC states show very distinct solvent responses, which are overlapping in time, as governed by the electronic dynamics. More broadly, this work demonstrates how VC/MM nonadiabatic dynamics simulations can resolve anisotropic solvent dynamics around a photoexcited solute with unprecedented detail, offering a new perspective that could stimulate the development of time-resolved experimental techniques capable of probing such solvent behaviour.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Resolving Femtosecond Solvent Reorganization Dynamics in an Iron Complex by Nonadiabatic Dynamics Simulations.J Am Chem Soc. 2022 Jul 20;144(28):12861-12873. doi: 10.1021/jacs.2c04505. Epub 2022 Jul 1. J Am Chem Soc. 2022. PMID: 35776920 Free PMC article.
-
Molecular feature-based classification of retroperitoneal liposarcoma: a prospective cohort study.Elife. 2025 May 23;14:RP100887. doi: 10.7554/eLife.100887. Elife. 2025. PMID: 40407808 Free PMC article.
-
Surveillance for Violent Deaths - National Violent Death Reporting System, 50 States, the District of Columbia, and Puerto Rico, 2022.MMWR Surveill Summ. 2025 Jun 12;74(5):1-42. doi: 10.15585/mmwr.ss7405a1. MMWR Surveill Summ. 2025. PMID: 40493548 Free PMC article.
-
Defining disease severity in atopic dermatitis and psoriasis for the application to biomarker research: an interdisciplinary perspective.Br J Dermatol. 2024 Jun 20;191(1):14-23. doi: 10.1093/bjd/ljae080. Br J Dermatol. 2024. PMID: 38419411 Free PMC article. Review.
References
-
- Jimenez R. Fleming G. R. Kumar P. V. Maroncelli M. Nature. 1994;369:471–473. doi: 10.1038/369471a0. - DOI
LinkOut - more resources
Full Text Sources

