Evidence that minocycline treatment confounds the interpretation of neurofilament as a biomarker
- PMID: 40417399
- PMCID: PMC12100619
- DOI: 10.1093/braincomms/fcaf175
Evidence that minocycline treatment confounds the interpretation of neurofilament as a biomarker
Abstract
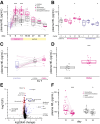
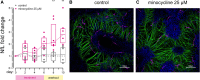
Neurofilament light (NfL) concentration in CSF and blood serves as an important biomarker in neurology drug development. Changes in NfL are generally assumed to reflect changes in neuronal damage, while little is known about the clearance of NfL from biofluids. In a study of asymptomatic individuals at risk for prion disease, both blood and CSF NfL spiked in one participant following a 6-week course of minocycline, absent any other biomarker changes and without subsequent onset of symptoms. We subsequently observed high NfL after minocycline treatment in discarded clinical plasma samples from inpatients, in mouse plasma and in conditioned media from neuron-microglia co-cultures. The specificity and kinetics of NfL response lead us to hypothesize that minocycline does not cause or exacerbate neuronal damage, but instead affects NfL by inhibiting its clearance, posing a potential confounder for the interpretation of this important biomarker.
Keywords: CSF; biomarker; minocycline; neurofilament.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
B.E., R.K., A.E. and C.B. are employees of IQ Proteomics. K.T.H. is an employee of Charles River Laboratories. S.E.A. acknowledges speaking fees from Abbvie, Biogen, EIP Pharma, Roche and Sironax; consulting fees from Athira, Biogen, Cassava, Cognito, Cortexyme, Sironax and vTv; and research support from Abbvie, Amylyx, EIP Pharma and Merck. E.V.M. acknowledges speaking fees from Eli Lilly; consulting fees from Deerfield and Alnylam; and research support from Ionis, Gate, Sangamo and Eli Lilly. H.Z. is co-founder of Brain Biomarker Solutions in Gothenburg AB and a GU Ventures-based platform company at the University of Gothenburg. S.M.V. acknowledges speaking fees from Ultragenyx, Illumina, Biogen and Eli Lilly; consulting fees from Invitae and Alnylam; and research support from Ionis, Gate, Sangamo and Eli Lilly.
Figures



Update of
-
Evidence that minocycline treatment confounds the interpretation of neurofilament as a biomarker.medRxiv [Preprint]. 2024 May 2:2024.05.01.24306384. doi: 10.1101/2024.05.01.24306384. medRxiv. 2024. Update in: Brain Commun. 2025 May 23;7(3):fcaf175. doi: 10.1093/braincomms/fcaf175. PMID: 38746398 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
