Long-term multi-meta-omics resolves the ecophysiological controls of seasonal N2O emissions during wastewater treatment
- PMID: 40417422
- PMCID: PMC12098122
- DOI: 10.1038/s44221-025-00430-x
Long-term multi-meta-omics resolves the ecophysiological controls of seasonal N2O emissions during wastewater treatment
Abstract
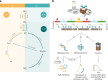
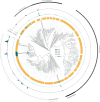
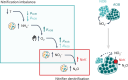
Nitrous oxide (N2O) is the third most important greenhouse gas and originates primarily from natural and engineered microbiomes. Effective emission mitigations are currently hindered by the largely unresolved ecophysiological controls of coexisting N2O-converting metabolisms in complex communities. To address this, we used biological wastewater treatment as a model ecosystem and combined long-term metagenome-resolved metaproteomics with ex situ kinetic and full-scale operational characterization over nearly 2 years. By leveraging the evidence independently obtained at multiple ecophysiological levels, from individual genetic potential to actual metabolism and emergent community phenotype, the cascade of environmental and operational triggers driving seasonal N2O emissions has ultimately been resolved. We identified nitrifier denitrification as the dominant N2O-producing pathway and dissolved O2 as the prime operational parameter, paving the way to the design and fostering of robust emission control strategies. This work exemplifies the untapped potential of multi-meta-omics in the mechanistic understanding and ecological engineering of microbiomes towards reducing anthropogenic impacts and advancing sustainable biotechnological developments.
Keywords: Climate-change ecology; Ecophysiology; Environmental biotechnology; Microbial ecology.
© The Author(s) 2025.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures




Similar articles
-
The impact of a seasonal change in loading rate on the nitrous oxide emissions at the WWTP of a tourist region.Sci Total Environ. 2022 Jan 15;804:149987. doi: 10.1016/j.scitotenv.2021.149987. Epub 2021 Aug 28. Sci Total Environ. 2022. PMID: 34517330
-
A decade of nitrous oxide (N2O) monitoring in full-scale wastewater treatment processes: A critical review.Water Res. 2019 Sep 15;161:392-412. doi: 10.1016/j.watres.2019.04.022. Epub 2019 Apr 14. Water Res. 2019. PMID: 31226538 Review.
-
Mitigating N2O emissions in land treatment systems: Mechanisms, influences, and future directions.Sci Total Environ. 2024 Nov 15;951:175638. doi: 10.1016/j.scitotenv.2024.175638. Epub 2024 Aug 20. Sci Total Environ. 2024. PMID: 39168319 Review.
-
Temporal triggers of N2O emissions during cyclical and seasonal variations of a full-scale sequencing batch reactor treating municipal wastewater.Sci Total Environ. 2021 Nov 25;797:149093. doi: 10.1016/j.scitotenv.2021.149093. Epub 2021 Jul 18. Sci Total Environ. 2021. PMID: 34303238
-
Nitrous oxide emissions and microbial communities variation in low dissolved oxygen and low carbon-to-nitrogen ratio anoxic-oxic wastewater treatment plant.Environ Sci Pollut Res Int. 2024 Jun;31(30):42779-42791. doi: 10.1007/s11356-024-33749-1. Epub 2024 Jun 15. Environ Sci Pollut Res Int. 2024. PMID: 38878241
References
-
- IPCC Climate Change 2014: Synthesis Report (eds Core Writing Team, Pachauri, R. K. & Meyer L. A.) (IPCC, 2014).
-
- Tian, H. et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature586, 248–256 (2020). - PubMed
-
- Vasilaki, V., Massara, T. M., Stanchev, P., Fatone, F. & Katsou, E. A decade of nitrous oxide (N2O) monitoring in full-scale wastewater treatment processes: a critical review. Water Res.161, 392–412 (2019). - PubMed
LinkOut - more resources
Full Text Sources
