Near-infrared fluorescent nanoprobe enables noninvasive, longitudinal monitoring of graft outcome in RPE transplantation
- PMID: 40417666
- PMCID: PMC12098337
- DOI: 10.3389/fmed.2025.1583790
Near-infrared fluorescent nanoprobe enables noninvasive, longitudinal monitoring of graft outcome in RPE transplantation
Abstract
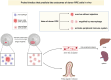
Objectives: Retinal pigment epithelium (RPE) cell transplantation holds therapeutic promise for retinal degenerative diseases, but longitudinal monitoring of graft survival and efficacy remains clinically challenging. The aim of this study is to develop a simple and effective method for the therapeutic quantification of RPE cell transplantation and immune rejection in vivo.
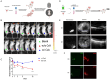
Methods: A nanoprobe was developed and modified to label donor RPE cells, and used to monitor the position and intensity of the fluorescence signal in vivo. Immunofluorescence staining and single-cell RNA sequencing (scRNA-seq) were used to characterize the cell types showing the fluorescence signal of the nanoprobe and to determine the composition of the immune microenvironment associated with subretinal transplantation.
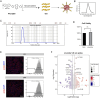
Results: The spatial distribution of the fluorescence signal of the nanoprobe corresponded with the site of transplantation, but the signal intensity decreased over time, while the signal distribution extended to the choroid. Additionally, the nanoprobe fluorescence signal was detected in the liver and spleen during long-term monitoring. Conversely, in mice administered the immunosuppressive drug cyclosporine A, the decrease in signal intensity was slower and the expansion of the signal distribution was less pronounced. Immunofluorescence analysis revealed a significant temporal increase in the proportion of macrophages with nanoprobe-labeled cells following transplantation. The stability and cell-penetrating ability of the nanoprobe enables the labeling of immune cell niches in RPE transplantation. Additionally, scRNA-seq analysis of nanoprobe-labeled cells identified MDK and ANXA1 signaling pathway in donor RPE cells as initiators of the immune rejection cascade, which were further amplified by macrophage-mediated pro-inflammatory signaling.
Conclusion: Near-infrared fluorescent nanoprobes represent a reliable method for in vivo tracing of donor RPE cells and long-term observation of nanoprobe distribution can be used to evaluate the degree of immune rejection. Molecular analysis of nanoprobe-labeled cells facilitates the characterization of the dynamic immune cell rejection niche and the landscape of donor-host interactions in RPE transplantation.
Keywords: RPE transplantation; immune rejection; in vivo tracking; macrophage; near-infrared fluorescent nanoprobe.
Copyright © 2025 Di, Lu, Xue, Zheng, Li, Xie, Yuan, Zhen, Wu, Mao and Yuan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The local and systemic secretion of the pro-inflammatory cytokine interleukin-6 after transplantation of retinal pigment epithelium cells in a rabbit model.Curr Eye Res. 2000 Jul;21(1):530-4. Curr Eye Res. 2000. PMID: 11035532
-
Near-infrared fluorescence 1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide (DiR)-labeled macrophages for cell imaging.2009 Dec 11 [updated 2010 Jan 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 Dec 11 [updated 2010 Jan 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641730 Free Books & Documents. Review.
-
Allogeneic iPSC-Derived RPE Cell Graft Failure Following Transplantation Into the Subretinal Space in Nonhuman Primates.Invest Ophthalmol Vis Sci. 2018 Mar 1;59(3):1374-1383. doi: 10.1167/iovs.17-22467. Invest Ophthalmol Vis Sci. 2018. PMID: 29625461 Free PMC article.
-
Donor Macrophages Modulate Rejection After Heart Transplantation.Circulation. 2022 Aug 23;146(8):623-638. doi: 10.1161/CIRCULATIONAHA.121.057400. Epub 2022 Jul 26. Circulation. 2022. PMID: 35880523 Free PMC article.
-
[Retinal pigment epithelial cell transplantation: perspective].Nippon Ganka Gakkai Zasshi. 1996 Dec;100(12):982-1006. Nippon Ganka Gakkai Zasshi. 1996. PMID: 9022310 Review. Japanese.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

