Broad-Spectrum Antimicrobial and Antibiofilm Activities of Halogenated Anilines Against Uropathogenic Escherichia coli and ESKAPE Pathogens
- PMID: 40417767
- PMCID: PMC12104821
- DOI: 10.1111/1751-7915.70165
Broad-Spectrum Antimicrobial and Antibiofilm Activities of Halogenated Anilines Against Uropathogenic Escherichia coli and ESKAPE Pathogens
Abstract
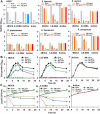
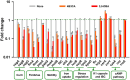
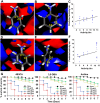
Uropathogenic Escherichia coli (UPEC) is one of the leading causes of nosocomial infections and urinary tract infections (UTIs), capable of inducing a spectrum of conditions ranging from acute bladder cystitis to chronic pyelonephritis. The virulence arsenal of UPEC includes factors, such as curli fimbriae, hemolysin, motility elements and metallophores. Moreover, UPEC can form biofilm-like communities and quiescent intracellular reservoirs within host tissues, contributing to recurrent and persistent infections. This study investigates the antibiofilm and antimicrobial activities of two halogen-substituted aniline derivatives, 4-bromo-3-chloroaniline (4B3CA) and 3,5-dibromoaniline (3,5-DBA), against UPEC. The compounds demonstrated minimum inhibitory concentrations (MICs) of 200 μg/mL for 4B3CA and 100 μg/mL for 3,5-DBA, with both exhibiting a biofilm inhibition IC50 value of 10 μg/mL. Additionally, these derivatives showed antibiofilm activity against ESKAPE pathogens. Treatment with 4B3CA and 3,5-DBA led to significant downregulation of UPEC virulence- and biofilm-related genes, including those involved in curli production, fimbrial adhesion, motility, iron acquisition, quiescent colony formation, and stress response. Interestingly, a mild upregulation of hlyA, csrA and uvrY was noted, alongside a marked downregulation of the adenylate cyclase genes cyaA and crp. These findings suggest that inhibition of adenylate cyclase activity may be a primary mode of action, leading to both antimicrobial and antibiofilm effects. The presence of halogen atoms in these compounds appears to enhance their binding affinity to adenylate cyclase through stabilising halogen bond interactions. Furthermore, 3D-QSAR analysis indicates that electrostatic favourability at the third and fourth positions of the aniline ring is critical for bioactivity. Finally, in silico ADMET profiling and cytotoxicity assessments using Caenorhabditis elegans suggest that these aniline derivatives hold promise as therapeutic candidates, warranting further investigation.
Keywords: E. coli; ESKAPE; UPEC; aniline; biofilm; halogen; virulence.
© 2025 The Author(s). Microbial Biotechnology published by John Wiley & Sons Ltd.
Conflict of interest statement
Declaration of Generative
The authors declare no conflicts of interest.
Figures




Similar articles
-
Antimicrobial and antibiofilm activities of chromone derivatives against uropathogenic Escherichia coli.Microbiol Res. 2024 Jan;278:127537. doi: 10.1016/j.micres.2023.127537. Epub 2023 Oct 27. Microbiol Res. 2024. PMID: 37922697
-
Purified α-Amylase from Bacillus cereus exhibits antibiofilm and antiquorum sensing activities against uropathogenic Escherichia coli, Downregulating fimH, and papC virulence genes: implications for urinary tract infections.BMC Microbiol. 2024 Nov 27;24(1):502. doi: 10.1186/s12866-024-03542-8. BMC Microbiol. 2024. PMID: 39604852 Free PMC article.
-
Antibiofilm and Antimicrobial Activities of Chloroindoles Against Uropathogenic Escherichia coli.Front Microbiol. 2022 Jun 16;13:872943. doi: 10.3389/fmicb.2022.872943. eCollection 2022. Front Microbiol. 2022. PMID: 35783430 Free PMC article.
-
A systematic review and meta-analysis of antibiotic resistance patterns, and the correlation between biofilm formation with virulence factors in uropathogenic E. coli isolated from urinary tract infections.Microb Pathog. 2020 Jul;144:104196. doi: 10.1016/j.micpath.2020.104196. Epub 2020 Apr 10. Microb Pathog. 2020. PMID: 32283258
-
Antibiotic Resistance Among Uropathogenic Escherichia coli.Pol J Microbiol. 2019 Dec;68(4):403-415. doi: 10.33073/pjm-2019-048. Epub 2019 Dec 5. Pol J Microbiol. 2019. PMID: 31880885 Free PMC article. Review.
References
-
- Atapalkar, R. S. , and Kulkarni A. A.. 2023. “Direct Amidation of Acids in a Screw Reactor for the Continuous Flow Synthesis of Amides.” Chemical Communications 59: 9231–9234. - PubMed
-
- Biville, F. , Oshima T., Mori H., et al. 2003. “ Escherichia coli Response to Exogenous Pyrophosphate and Analogs.” Journal of Molecular Microbiology and Biotechnology 5, no. 1: 37–45. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

