Initiating Empagliflozin and Sacubitril/Valsartan Early After Acute Myocardial Infarction: Mechanistic Study
- PMID: 40417813
- PMCID: PMC12229191
- DOI: 10.1161/JAHA.124.040214
Initiating Empagliflozin and Sacubitril/Valsartan Early After Acute Myocardial Infarction: Mechanistic Study
Abstract
Background: Empagliflozin and sacubitril/valsartan are established in heart failure treatment, but their effects after myocardial infarction (MI) are less clear. This study evaluated early empagliflozin initiation, with or without sacubitril/valsartan, on post-MI inflammation, oxidative stress, metabolism, fibrosis, cardiac function, and ventricular tachycardia (VT) risk in a pig model.
Methods: A total of 24 of 30 pigs survived the MI procedure and were subsequently randomized to receive beta-blocker treatment alone (control-MI), beta-blocker+empagliflozin, or beta-blocker+empagliflozin+sacubitril/valsartan. Immune response, metabolic profile, and cardiac function were monitored. At 30 days after MI, programmed electrical stimulation and high-density mapping were performed and VT inducibility was assessed. Tissue samples were collected for cardiac inflammation, oxidative stress, and metabolic analyses.
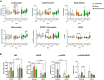
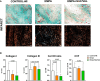
Results: Empagliflozin reduced circulating leukocytes at 2 and 15 days after MI (P=0.010 and P=0.050, respectively) and decreased C-C chemokine receptor 2+ monocytes at 15 days (P=0.049). Nitric oxide bioavailability increased in remote myocardium (P=0.059), along with cardioprotective liver lipids and collagen III in the myocardial scar (P=0.023). No effect on cardiac function or VT inducibility was observed at 30 days. With empagliflozin+sacubitril/valsartan, scar collagen I decreased (P=0.082), left ventricular compliance improved (P=0.029), electrophysiological remodeling improved (reduced border-zone corridors [P=0.006] and deceleration zones [P=0.008]), and VT inducibility decreased (P=0.025).
Conclusions: In this pig model of nonreperfused MI treated with beta-blocker, early initiation of empagliflozin reduced inflammation, improved nitric oxide bioavailability, increased protective liver lipids, and modified scar composition without affecting cardiac function or VT risk. With empagliflozin+sacubitril/valsartan treatment, scar collagen I and VT inducibility declined and left ventricular remodeling was enhanced.
Keywords: empagliflozin; fibrosis; inflammation; myocardial infarction; sacubitril/valsartan; ventricular arrhythmia.
Conflict of interest statement
This work was partially funded by Boehringer Ingelheim through an unrestricted grant with Germans Trias i Pujol Health Science Research Institute. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations in relation to potential mention of Boehringer Ingelheim substances.
Dr Delgado has received speaker fees from Abbott Vascular, Edwards Lifesciences, GE Healthcare, Medtronic, Novartis, JenaValve, and Philips and consulting fees from Edwards Lifesciences, MSD, and Novo Nordisk. Dr Bayes‐Genis has lectured and/or participated in ad boards for Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Novo Nordisk, Roche Diagnostics, and Vifor. Dr Bisbal has received speaker fees from Abbott, Biosense Webster, and Biotronik and consulting fees from Abbott. Dr Gálvez‐Montón is a cofounder and the CSO of NIMBLE Diagnostics. Dr Ferrer‐Curriu has received fees from Boehringer Ingelheim. Núria Amigó is a stock owner of Biosfer Teslab and has a patent on the method for lipoprotein profiling described in the present article.
Figures


References
-
- Lippi G, Sanchis‐Gomar F. Global epidemiology and future trends of heart failure. AME Med J. 2020;5:1–6. doi: 10.21037/amj.2020.03.03 - DOI
-
- Gerber Y, Weston SA, Enriquez‐Sarano M, Berardi C, Chamberlain AM, Manemann SM, Jiang R, Dunlay SM, Roger VL. Mortality associated with heart failure after myocardial infarction: a contemporary community perspective. Circ Heart Fail. 2016;9:e002460. doi: 10.1161/CIRCHEARTFAILURE.115.002460 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

