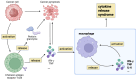
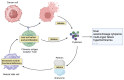
Chimeric antigen receptor T cell immunotherapy‑associated hemophagocytic lymphohistiocytosis: Pathogenesis, clinical manifestation, diagnosis and management compared with cytokine release syndrome (Review)
- PMID: 40417883
- PMCID: PMC12134842
- DOI: 10.3892/mmr.2025.13578
Chimeric antigen receptor T cell immunotherapy‑associated hemophagocytic lymphohistiocytosis: Pathogenesis, clinical manifestation, diagnosis and management compared with cytokine release syndrome (Review)
Abstract
Chimeric antigen receptor (CAR) T cell therapy is used to treat hematological malignancy. However, it carries the risk of life‑threatening inflammatory toxicity, including cytokine release syndrome (CRS) and CAR T cell‑associated hemophagocytic lymphohistiocytosis (CARHLH). CRS is a common side effect of CAR T cell therapy, with fever and multiorgan functional impairment as the primary clinical manifestation. CARHLH and CRS have similar clinical manifestations. However, CARHLH is associated with a high mortality rate. CARHLH was previously considered a specific type of CRS, however, it must be promptly differentiated from CRS for treatment initiation, with management differing from that of CRS. The pathogenesis of CARHLH differs from that of CRS. The present review aimed to summarize the pathogenesis, diagnosis and treatment of CARHLH to assist in its early identification and management.
Keywords: chimeric antigen receptor T cell immunotherapy; chimeric antigen receptor T cell‑associated hemophagocytic lymphohistiocytosis; cytokine release syndrome; diagnosis; hemophagocytic lymphohistiocytosis; treatment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
A distinct cytokine network distinguishes chimeric antigen receptor T cell (CAR-T)-associated hemophagocytic lymphohistiocytosis-like toxicity (carHLH) from severe cytokine release syndrome following CAR-T therapy.Cytotherapy. 2023 Nov;25(11):1167-1175. doi: 10.1016/j.jcyt.2023.06.008. Epub 2023 Jul 21. Cytotherapy. 2023. PMID: 37480884
-
Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T cells.Blood. 2021 Dec 16;138(24):2469-2484. doi: 10.1182/blood.2021011898. Blood. 2021. PMID: 34525183 Free PMC article. Clinical Trial.
-
Latest updates on pathogenesis mechanisms and management strategies for cytokine release syndrome, neurotoxicity, and hemophagocytic lymphohistiocytosis related to CAR-T cell therapies.Ann Hematol. 2025 Jun;104(6):3129-3151. doi: 10.1007/s00277-025-06467-y. Epub 2025 Jun 19. Ann Hematol. 2025. PMID: 40536724 Free PMC article. Review.
-
Hemophagocytic lymphohistiocytosis-like toxicity (carHLH) after CD19-specific CAR T-cell therapy.Br J Haematol. 2021 Aug;194(4):701-707. doi: 10.1111/bjh.17662. Epub 2021 Jul 15. Br J Haematol. 2021. PMID: 34263927 Free PMC article.
-
[The diagnosis and treatment of chimeric antigen receptor T-cell associated hemophagocytic lymphohistiocytosis].Zhonghua Nei Ke Za Zhi. 2025 Jan 1;64(1):77-82. doi: 10.3760/cma.j.cn112138-20240506-00284. Zhonghua Nei Ke Za Zhi. 2025. PMID: 39788599 Review. Chinese.
References
-
- Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources