Exploring metal bioaccumulation ability of boreal white-rot fungi on fiberbank material
- PMID: 40418047
- PMCID: PMC12118429
- DOI: 10.1080/21655979.2025.2507539
Exploring metal bioaccumulation ability of boreal white-rot fungi on fiberbank material
Abstract
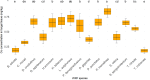
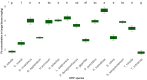
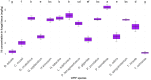
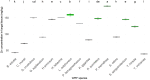
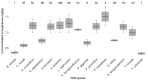
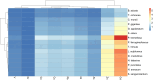
Fiberbanks are organic-rich sediment deposits in aquatic environments, primarily formed through historical pulp and paper mill activities. These deposits consist of wood-derived fibrous materials and are contaminated with potentially toxic elements (PTEs) such as vanadium, chromium, cobalt, nickel, copper, zinc, arsenic, cadmium, and lead. The leaching of these contaminants into surrounding waters poses significant environmental and health risks, impacting aquatic ecosystems and potentially entering the food chain. Effective remediation of fiberbanks is crucial, particularly in Sweden and other regions with extensive wood-pulping industries. This study aims to evaluate the bioaccumulation capacities of 26 native Swedish white-rot fungi (WRF) species for the remediation of PTEs in fiberbank material. Fiberbank samples were collected from Sundsvall's Bay in the Baltic Sea, while the fungal species were isolated from boreal forests in Västernorrland, Sweden. The fungi were cultured on Hagem agar medium with sterilized fiberbank material as the substrate. After two months, fungal biomass was analyzed for PTE uptake using inductively coupled plasma-mass spectrometry (ICP-MS). The results revealed significant variability (p < 0.001) in PTE uptake among fungal species. Phlebia tremellosa consistently demonstrated the highest bioconcentration factors for analyzed elements, with values for V (0.39), Cr (0.10), Co (1.81), Cu (1.54), Pb (1.65), Ni (1.28), As (0.83), Zn (3.61), and Cd (5.56). Other species, including Laetiporus sulphureus (0.09-4.78), Hymenochaete tabacina (0.08-4.52), and Diplomitoporus crustulinus (0.08-4.48), also exhibited significant bioremediation potential. These findings highlight the potential of native WRF species for PTEs remediation in fiberbanks and provide a foundation for mycoremediation strategies in contaminated environments.
Keywords: Bioremediation; fiberbank; heavy metals; mycoremediation; potentially toxic elements; white-rot fungi.
© 2025 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures







References
-
- Dahlberg AK, Apler A, Vogel L, et al. Persistent organic pollutants in wood fiber–contaminated sediments from the Baltic Sea. J Soils Sediments. 2020;20(5):2471–17. doi: 10.1007/s11368-020-02610-6 - DOI
-
- Göransson G, Apler A, Dahlberg AK, et al. Assessing the risk of contaminant dispersion from fibrous sediments of industrial origin. Front Mar Sci. 2021;8:729243. doi: 10.3389/fmars.2021.729243 - DOI
-
- Lehoux AP, Isidorova A, Collin F, et al. Extreme gas production in anthropogenic fibrous sediments: an overlooked biogenic source of greenhouse gas emissions. Sci Total Environ. 2021;781:146772.
-
- Haller H, Paladino G, Dupaul G, et al. Polluted lignocellulose-bearing sediments as a resource for marketable goods—a review of potential technologies for biochemical and thermochemical processing and remediation. Clean Technol Environ Policy. 2023;25(2):409–425.
-
- Löfroth H, O’Regan M, Snowball I, et al. Challenges in slope stability assessment of contaminated fibrous sediments along the northern Baltic coast of Sweden. Eng Geol. 2021;289:106190. doi: 10.1016/j.enggeo.2021.106190 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
