Adaptive thermogenesis driving catch-up fat during weight regain: a role for skeletal muscle hypothyroidism and a risk for sarcopenic obesity
- PMID: 40418496
- PMCID: PMC12534267
- DOI: 10.1007/s11154-025-09970-9
Adaptive thermogenesis driving catch-up fat during weight regain: a role for skeletal muscle hypothyroidism and a risk for sarcopenic obesity
Abstract
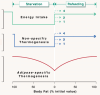
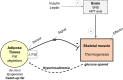
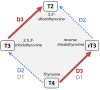
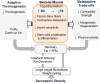
Across the spectrum of weight regain, ranging from cachexia rehabilitation and catch-up growth to obesity relapse, the recovery rate of body fat is often disproportionate relative to lean tissue recovery. Such preferential 'catch-up fat' is in part attributed to an increase in metabolic efficiency and embodied in the concept that 'metabolic adaptation' or 'adaptive thermogenesis' in response to large weight deficits can persist during weight regain to accelerate fat stores recovery. This paper reviews the evidence in humans for the existence of this thrifty metabolism driving catch-up fat within the framework of a feedback loop between fat stores depletion and suppressed thermogenesis. The search for its effector mechanisms suggests that whereas adaptive thermogenesis during weight loss results primarily from central suppression of sympathetic nervous system and hypothalamic-pituitary-thyroid axis, its persistence during weight regain for accelerating fat recovery is primarily mediated through peripheral tissue resistance to the actions of this systemic neurohormonal network. Emerging evidence linking it to an upregulation of skeletal muscle type 3 deiodinase (D3), the main thyroid hormone inactivating enzyme, along with slowed muscle metabolism and altered contractile properties, suggest that D3-induced muscle hypothyroidism is a key feature of such peripheral resistance. These findings underlying a role of skeletal muscle hypothyroidism in adaptive thermogenesis driving catch-up fat, but which can also concomitantly compromise muscle functionality, have been integrated into a mechanistic framework to explain how weight cycling and large weight fluctuations across the lifespan can predispose to sarcopenic obesity.
Keywords: Catch-up growth; Deiodinases; Metabolic adaptation; Obesity; Thyroid hormones; Weight cycling.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- World Health Organization. Obesity. https://www.who.int/health-topics/obesity#tab=tab_1 (accessed April 05, 2025).
-
- Bray GA, Kim KK, Wilding JPH, World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the world obesity federation. Obes Rev. 2017;18(7):715–23. 10.1111/obr.12551. - PubMed
-
- Halpern B, Mancini MC, de Melo ME, Lamounier RN, Moreira RO, Carra MK, et al. Proposal of an obesity classification based on weight history: an official document by the Brazilian society of endocrinology and metabolism (SBEM) and the Brazilian society for the study of obesity and metabolic syndrome (ABESO). Arch Endocrinol Metab. 2022;66(2):139–51. 10.20945/2359-3997000000465. - PMC - PubMed
-
- Busetto L, Dicker D, Frühbeck G, Halford JCG, Sbraccia P, Yumuk V, et al. A new framework for the diagnosis, staging and management of obesity in adults. Nat Med. 2024;30(9):2395–9. 10.1038/s41591-024-03095-3. - PubMed
-
- Dulloo AG, Jacquet J, Solinas G, Montani JP, Schutz Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes (Lond). 2010;34(Suppl 2):S4–17. 10.1038/ijo.2010.234. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

