Accumulation of GSK-3β in Interneurons Impairs Adult Hippocampal Neurogenesis by Inhibiting GABAergic Transmission
- PMID: 40418513
- PMCID: PMC12341769
- DOI: 10.1111/acel.70115
Accumulation of GSK-3β in Interneurons Impairs Adult Hippocampal Neurogenesis by Inhibiting GABAergic Transmission
Abstract
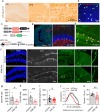
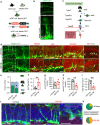
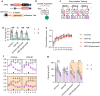
The activation of glycogen synthase kinase 3β (GSK-3β) and the deterioration of spatial memory represent prominent pathological and clinical manifestations of Alzheimer's disease (AD). Nevertheless, the precise intrinsic mechanisms linking these pathological features remain poorly elucidated. In this study, we identified significant upregulation of GSK-3β activity in inhibitory interneurons within the hippocampal dentate gyrus (DG) of 3×Tg-AD mice. Subsequent investigations demonstrated that targeted overexpression of GSK-3β in these interneurons triggered aberrant activation of neural stem cells (NSCs), culminating in apoptotic cell death and consequent deficits in adult hippocampal neurogenesis (AHN). Utilizing in vivo fiber-optic recording techniques, we further established that GSK-3β overexpression in DG inhibitory interneurons elicited hyperactivation of excitatory neurons, thereby disrupting the excitation-inhibition (E/I) balance within the DG circuitry. Notably, these pathological alterations were ameliorated through chemogenetic suppression of excitatory neuronal activity. Mechanistically, we determined that impaired GABAergic transmission, characterized by reduced GABA release in the DG region, underlies these observed effects. Pharmacological intervention with GABA receptor agonists effectively rescued AHN impairment and attenuated spatial cognitive deficits. Collectively, these findings demonstrate that GSK-3β overexpression in GABAergic interneurons compromises AHN and promotes NSC apoptosis via disruption of GABAergic signaling, while pharmacological potentiation of GABAergic transmission exerts neuroprotective effects. This study elucidates a previously unrecognized mechanism contributing to AHN impairment in AD and identifies a promising therapeutic target for pro-neurogenic strategies.
Keywords: GABA transmission; adult hippocampal neurogenesis; glycogen synthase kinase‐3β; interneuron.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

