The burden, pathogenesis, clinical outcomes, and treatment of common respiratory virus infections during pregnancy
- PMID: 40418749
- PMCID: PMC12106989
- DOI: 10.1177/17455057251338501
The burden, pathogenesis, clinical outcomes, and treatment of common respiratory virus infections during pregnancy
Abstract
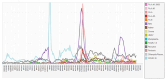
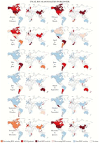
Respiratory illnesses due to respiratory virus infections disproportionately impact pregnant individuals and their infants, leading to significant morbidity and mortality globally. Data describing the incidence and impact of these infections in pregnancy is sparse and more common for influenza and now severe acute respiratory syndrome coronavirus 2 with less data available on other respiratory virus infections in pregnancy. This lack of data is a result of limited prospective surveillance and issues surrounding the calculations of seroprevalence, as well as disproportionately low funding for reproductive health research. In this review article, we aimed to summarize available data on respiratory virus infections in pregnancy and identify gaps in the published literature.
Keywords: infection; neonate; pregnancy; respiratory virus.
Plain language summary
Respiratory virus infections, like flu and COVID-19, cause serious illness and death in pregnant people and their babies, worldwide. However, there’s not much data on how common these infections are or their full impact during pregnancy, especially for viruses other than flu and COVID-19. This is due to limited ongoing studies, challenges in measuring past infections, and underfunding of reproductive health research. This article reviews what we know about these infections in pregnancy and points out where more research is needed.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors do not have competing interests to disclose related to this publication. Outside of this work, A.K. was an unpaid consultant for Pfizer and GlaxoSmithKline and is a co-investigator on studies funded by Merck and Pfizer. J.A.E. also receives grant support to her institution from Merck, GlaxoSmithKline, AstraZeneca, and Pfizer, and is a consultant for AbbVie, Ark Biopharma, AstraZeneca, GlaxoSmithKline, Moderna, Pfizer, Sanofi Pasteur, and Meissa Vaccines, Inc. outside of the described work.
Figures

References
-
- Takeda S, Hisano M, Komano J, et al.. Influenza vaccination during pregnancy and its usefulness to mothers and their young infants. J Infect Chemother 2015; 21(4): 238–246. - PubMed
-
- Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 2017; 17(8): 469–482. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

