5-HT2A receptors: Pharmacology and functional selectivity
- PMID: 40418878
- PMCID: PMC12405928
- DOI: 10.1016/j.pharmr.2025.100059
5-HT2A receptors: Pharmacology and functional selectivity
Abstract
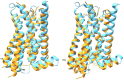
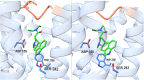
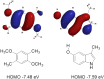
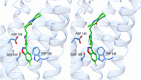
Serotonin 5-HT2A receptors were one of the first serotonin receptors to be pharmacologically characterized. In mammals, they are expressed throughout the body in nearly every cell and tissue type, with the highest density in cortical layer V of the brain. They are involved in several aspects of normal physiological processes and behaviors and have been implicated in the etiology of neuropsychiatric diseases such as schizophrenia. Atypical antipsychotics have targeted blockade of 5-HT2A receptors as part of their therapeutic mechanism. More recently, 5-HT2A receptors have come to prominence for their role as the primary target for psychedelic drugs, which activate this receptor subtype to produce their characteristic behavioral effects. 5-HT2A receptor agonists like psilocybin, dimethyltryptamine, and lysergic acid diethylamide have each demonstrated long-lasting therapeutic efficacy in clinical trials for psychiatric disorders such as major depression and substance use disorders. There is a significant effort in both academia and industry to develop new agonists of 5-HT2A receptors with therapeutic efficacy. There are 3 primary scaffolds for agonists: tryptamines, ergolines, and phenylalkylamines, each engaging different subsets of amino acid residues in the receptor binding pocket. Differences can lead to differential responses between ligands for functionally selective outcomes. Here, we provide a historical perspective on 5-HT2A receptors, their key structural features and motifs involved in ligand-receptor interactions, and how these interactions can affect signaling pathways downstream of the receptor. Understanding how ligands interact with the 5-HT2A receptor will fundamentally inform future drug discovery to optimize therapeutics for a variety of disorders. SIGNIFICANCE STATEMENT: Psychedelic drugs have demonstrated long-lasting therapeutic efficacy for several conditions in multiple clinical trials. Their target, serotonin 5-HT2A receptors, are GPCRs with complex pharmacology. Having knowledge of how ligands interact with 5-HT2A receptors in the orthosteric binding pocket at the structural level to induce specific signal transduction pathways will inform on efforts to design and develop functionally selective drugs to potentially treat a variety of diseases.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest Charles D. Nichols is a founder of and member of the Board of Directors for 2A Biosciences, has a sponsored research agreement with 2A Biosciences, and is a consultant for Palo Santo VC. David E. Nichols is a founder and employee of 2A Biosciences. Gerald B. Billac is an employee of 2A Biosciences. The other author declares no conflicts of interest.
Figures




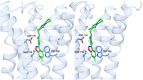

References
-
- Affolter H., Erne P., Bürgisser E., Pletscher A. Ca2+ as messenger of 5HT2-receptor stimulation in human blood platelets. Naunyn Schmiedebergs Arch Pharmacol. 1984;325:337–342. - PubMed
-
- Almaula N., Ebersole B.J., Zhang D., Weinstein H., Sealfon S.C. Mapping the binding site pocket of the serotonin 5-hydroxytryptamine2A receptor. Ser3.36(159) provides a second interaction site for the protonated amine of serotonin but not of lysergic acid diethylamide or bufotenin. J Biol Chem. 1996;271:14672–14675. - PubMed
-
- Bennett J.P., Snyder S.H. Serotonin and lysergic acid diethylamide binding in rat brain membranes: relationship to postsynaptic serotonin receptors. Mol Pharmacol. 1976;12:373–389. - PubMed
-
- Berg K.A., Clarke W.P., Chen Y., Ebersole B.J., McKay R.D., Maayani S. 5-Hydroxytryptamine type 2A receptors regulate cyclic AMP accumulation in a neuronal cell line by protein kinase C-dependent and calcium/calmodulin-dependent mechanisms. Mol Pharmacol. 1994;45:826–836. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

