JNK1 in SF1 neurons regulates the central action of thyroid hormones on hepatic lipid metabolism
- PMID: 40419019
- PMCID: PMC12173623
- DOI: 10.1016/j.molmet.2025.102170
JNK1 in SF1 neurons regulates the central action of thyroid hormones on hepatic lipid metabolism
Abstract
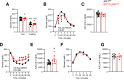
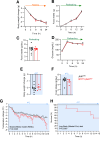
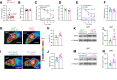
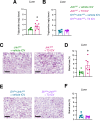
Objective: Hypothalamic energy sensors, such as AMP-activated protein kinase (AMPK), and stress sensors, such as c-Jun N-terminal kinase 1 (JNK1, also known as MAPK8) modulate whole body energy balance. While the role of AMPK in steroidogenic factor 1 (SF1) neurons of the VMH has been investigated, the relevance of JNK1 in this neuronal population has not been addressed. Here, we investigated the involvement of JNK1 SF1 on energy homeostasis.
Methods: We generated mice bearing conditional JNK1 disruption through Mapk8 gene deletion in SF1 neurons (Sf1Cre/Jnk1fl/fl). Complete metabolic phenotyping, fasting/refeeding and cold challenges, as well as the central response to triiodothyronine (T3) on brown adipose tissue (BAT) thermogenesis and hepatic lipid metabolism were carried out.
Results: Sf1Cre/Jnk1fl/fl mice displayed decreased body weight, improved glucose tolerance, and reduced hepatic lipid levels. However, Sf1Cre/Jnk1fl/fl did not properly defend their temperature upon cold exposure. While central administration of T3 elicited feeding independent weight loss in both wildtype (Jnk1fl/fl) and SF1Cre/Jnk1fl/fl mice, it did not promote hepatic lipid accretion in null animals.
Conclusions: Our data demonstrated for the first time that JNK1 in SF1 neurons is necessary for the regulation of hepatic lipid metabolism, cold adaptation and central T3 actions.
Keywords: BAT; Hypothalamus; JNK1; Liver; SF1; T3.
Copyright © 2025 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest Miguel López serves as Scientific Director of Gazella Biotech (https://gazellabiotech.com/). Rubén Nogueiras serves in the Advisory Board of Albor Biotech (https://alborbiotech.com/). The rest of the authors have no other conflicts of interest.
Figures




References
-
- Dragano N.R.V., Ferno J., Dieguez C., Lopez M., Milbank E. Recent updates on obesity treatments: available drugs and future directions. Neuroscience. 2020;447:191–215. - PubMed
-
- López M., Nogueiras R., Tena-Sempere M., Dieguez C. Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nat Rev Endocrinol. 2016;12(7):421–432. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

