Reverse-normal immunopurification: An effective approach for purifying recombinant erythropoietin from its analogues in doping analysis
- PMID: 40419139
- PMCID: PMC12270052
- DOI: 10.1016/j.jshs.2025.101062
Reverse-normal immunopurification: An effective approach for purifying recombinant erythropoietin from its analogues in doping analysis
Abstract
Background: Recombinant erythropoietin (rEPO) is commonly used in therapy but may be abused in sports to enhance endurance. In doping analysis, rEPO can be detected in human urine or blood samples at picogram (pg) levels based on its slightly higher molecular weight (MW) than that of endogenous EPO using western blotting (WB). However, a type of variant erythropoietin (VAR-EPO) encoded by the EPO c.577del variant has a similar MW to rEPO, and these 2 molecules cannot be distinguished using conventional analytical methods. A fit-for-purpose method needs to be developed immediately.
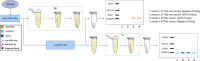
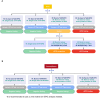
Methods: In this study, we introduced a reverse-normal immunopurification technique for sample pretreatment to remove VAR-EPO from samples to eliminate its interference with rEPO detection. Firstly, a rabbit monoclonal antibody (mAb) that can specifically recognize trace amounts of VAR-EPO with high affinity was generated. Then, using this antibody to enrich VAR-EPO, we developed reverse-normal immunopurification coupled with WB on the purpose of analyzing rEPO in urine and serum samples. Next, the method was fully validated and evaluated using blank samples, spiked samples and rEPO excreted samples. Finally, the identification criteria of rEPO was established.
Results: A specific anti-VAR mAb with high affinity was developed. Using it, we developed the doping analytical method for rEPO. Our method effectively detects and removes VAR-EPO, enabling accurate rEPO detection.
Conclusion: A method has already been applied for rEPO confirmation in routine doping analyses.
Keywords: Doping analysis; Erythropoietin; Immunopurification; Western blotting.
Copyright © 2025. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Study on Internal Standards Applicable to the Detection of Recombinant Erythropoietin.Drug Test Anal. 2025 Aug;17(8):1294-1302. doi: 10.1002/dta.3836. Epub 2024 Dec 5. Drug Test Anal. 2025. PMID: 39632780
-
A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment.Health Technol Assess. 2007 Apr;11(13):1-202, iii-iv. doi: 10.3310/hta11130. Health Technol Assess. 2007. PMID: 17408534
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Frequency of administration of recombinant human erythropoietin for anaemia of end-stage renal disease in dialysis patients.Cochrane Database Syst Rev. 2002;(4):CD003895. doi: 10.1002/14651858.CD003895. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003895. doi: 10.1002/14651858.CD003895.pub2. PMID: 12519614 Updated.
References
-
- Jelkmann W. Developments in the therapeutic use of erythropoiesis stimulating agents. Br J Haematol. 2008;141:287–297. - PubMed
-
- Eschbach J.W., Egrie J.C., Downing M.R., Browne J.K., Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. N Engl J Med. 1987;317:249–251. - PubMed
-
- Ohls RK. Human recombinant erythropoietin in the prevention and treatment of anemia of prematurity. Paediatr Drugs. 2002;4:111–121. - PubMed
-
- Crawford J. Recombinant human erythropoietin in cancer-related anemia. Oncology (Williston Park) 2002;16:41–53. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

