Effect of Chalcogen Interaction on the Structure of Methine-Bridged Trichalcogenophenes
- PMID: 40419448
- PMCID: PMC12271992
- DOI: 10.1002/chem.202501123
Effect of Chalcogen Interaction on the Structure of Methine-Bridged Trichalcogenophenes
Abstract
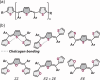
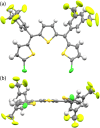
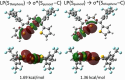
Polythienylenemethylidenes (PTMs) are promising conjugated polymers for organic electronics owing to their narrow bandgaps and extended π-conjugation. However, their stereochemistry remains unexplored. In this study, methine-bridged trithiophene and trifuran analogs were synthesized to investigate stereochemistry and chalcogen bonding effects. The compounds were obtained as mixtures of ZZ, EZ(= ZE), and EE geometric isomers, established through detailed NMR analyses. At thermal equilibrium, the ZZ isomer predominated in trithiophene (ZZ:(EZ + ZE):EE = 58:35:6), whereas trifuran showed a near-statistical distribution. X-ray crystallography revealed intramolecular S···S chalcogen bonding in trithiophene with S···S distances (≈3.04 Å) shorter than van der Waals radii and C-S···S angles of 171°. Comprehensive conformer searches and DFT calculations not only validated the higher stability of the ZZ isomer in trithiophene but also provided calculated isomer distributions that closely matched the experimental values. Multi-faceted computational analysis (electron localization function (ELF), noncovalent interaction (NCI), quantum theory of atoms in molecules (QTAIM), and natural bond orbital (NBO)) confirmed the presence of these chalcogen-centered interactions and quantified their strength through lone pair (LP)(S)→σ*(S-C) donor-acceptor orbital interactions. Trithiophene exhibited a unique dual-chalcogen bonding mode in the ZZ configuration. These findings elucidate the role of chalcogen bonding in stabilizing ZZ-trithiophenes and contribute to designing PTMs with controlled stereochemistry for organic electronics applications.
Keywords: chalcogen bonding; conjugation; diastereoselectivity; noncovalent interactions; thiophene.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Roncali J., Chem. Rev. 1997, 97, 173. - PubMed
-
- Pron A., Rannou P., Prog. Polym. Sci. 2002, 27, 135.
-
- Beaujuge P. M., Reynolds J. R., Chem. Rev. 2010, 110, 268. - PubMed
-
- Guo X., Baumgarten M., Müllen K., Prog. Polym. Sci. 2013, 38, 1832.
-
- Dou L., Liu Y., Hong Z., Li G., Yang Y., Chem. Rev. 2015, 115, 12633. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

