Cortical circuits for cross-modal generalization
- PMID: 40419471
- PMCID: PMC12106601
- DOI: 10.1038/s41467-025-59342-9
Cortical circuits for cross-modal generalization
Abstract
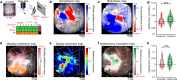
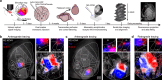
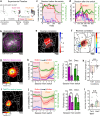
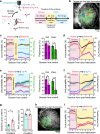
Adapting goal-directed behaviors to changing sensory conditions is a fundamental aspect of intelligence. The brain uses abstract representations of the environment to generalize learned associations across sensory modalities. The circuit organization that mediates such cross-modal generalizations remains, however, unknown. Here, we demonstrate that mice can bidirectionally generalize sensorimotor task rules between touch and vision by using abstract representations of peri-personal space within the cortex. Using large-scale mapping in the dorsal cortex at single-cell resolution, we discovered multimodal neurons with congruent spatial representations within multiple associative areas of the dorsal and ventral streams. Optogenetic sensory substitution and systematic silencing of these associative areas revealed that a single area in the dorsal stream is necessary and sufficient for cross-modal generalization. Our results identify and comprehensively describe a cortical circuit organization that underlies an essential cognitive function, providing a structural and functional basis for abstract reasoning in the mammalian brain.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Behrens, T. E. J. et al. What is a cognitive map? organizing knowledge for flexible behavior. Neuron100, 490–509 (2018). - PubMed
-
- Cloke, J. M., Jacklin, D. L. & Winters, B. D. The neural bases of crossmodal object recognition in non-human primates and rodents: A review. Behav. Brain Res.285, 118–130 (2015). - PubMed
-
- Davenport, R. K. & Rogers, C. M. Intermodal equivalence of stimuli in apes. Science (1979)168, 279–280 (1970). - PubMed
-
- Solvi, C., Gutierrez Al-Khudhairy, S. & Chittka, L. Bumble bees display cross-modal object recognition between visual and tactile senses. Science (1979)367, 910–912 (2020). - PubMed
-
- Bruck, J. N. & Pack, A. A. Understanding across the senses: cross-modal studies of cognition in cetaceans. Anim. Cogn.25, 1059–1075 (2022). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

