Attrition rates and treatment outcomes in multiple myeloma: real-world data over a 40-year period
- PMID: 40419498
- PMCID: PMC12106733
- DOI: 10.1038/s41408-025-01311-y
Attrition rates and treatment outcomes in multiple myeloma: real-world data over a 40-year period
Abstract
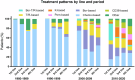
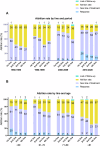
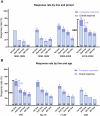
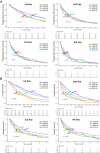
The treatment landscape of multiple myeloma (MM) has evolved significantly over four decades, driven by novel therapies and optimized supportive care. However, the attrition rate (AR), defined as the proportion of patients who die without advancing to the next line of therapy (LOT) after treatment failure, remains a major challenge. To assess how treatment patterns and outcomes have evolved, we analyzed 1,297 MM patients treated between 1980 and 2020, stratified by diagnosis period and age. ARs declined from 38-55% in the 1980s to 15-20% in 2010-2020, but remained high in older patients, with 46.9% of those over 80 unable to proceed beyond first LOT. While progression-free survival gains were primarily observed in the first LOT (15.8 to 24.1 months, p = 0.001), overall survival (OS) improved across all LOTs and age groups, likely due to more effective salvage therapies and supportive care. Achieving a complete response in first-line therapy was associated with a significant OS benefit (4.5 vs. 1.6 years, p < 0.001), underscoring its importance, as many patients, particularly older ones, are less likely to reach subsequent LOTs. Despite advances in MM treatment, patient loss to attrition remains a challenge, highlighting the need for more effective therapies early in the disease course.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: LGRL: Honoraria and travel grants from Janssen, Amgen, GSK, BMS, Sanofi, and Menarini Stemline; AD: Honoraria from Janssen, travel grants from Sanofi, Janssen, Binding Side; DFM: Honoraria and travel grants from Janssen; MTC: Honoraria from Janssen, Sanofi, GSA, Amgen, and Pfizer, LR: Honoraria from BMS, Celgene, Amgen, Takeda, Sanofi, Janssen, GSK; JB: Honoraria from Janssen, Celgene, BMS, Amgen, Takeda, Oncopeptides; CFL: Consultancy: BeiGene, Sanofi, GSK, BMS, Janssen; Honoraria: BeiGene, Pfizer, Sanofi, GSK, Takeda, BMS, Janssen; Research Funding: Takeda, Amgen, BMS, Janssen. The rest of the authors declare no competing interests. Ethics approval and consent to participate: All methods were performed in accordance with the relevant guidelines and regulations, including the Declaration of Helsinki. The study was approved by the Ethics Committee of the Hospital Clínic de Barcelona. Informed consent was obtained from all participants, authorizing the use of their anonymized clinical data for research purposes.
Figures

References
MeSH terms
Grants and funding
- FIS PI22/00647/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- ICI19/00025/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- 2021SGR01292/Generalitat de Catalunya (Government of Catalonia)
- LABAE21971FERN/Fundación Científica Asociación Española Contra el Cáncer (Scientific Foundation, Spanish Association Against Cancer)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

