An antigen panel to assess the regional relevance of foot and mouth disease vaccines
- PMID: 40419503
- PMCID: PMC12106609
- DOI: 10.1038/s41541-025-01128-7
An antigen panel to assess the regional relevance of foot and mouth disease vaccines
Abstract
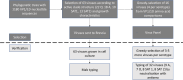
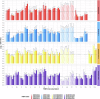
Despite widespread use of inactivated vaccines to control foot-and-mouth disease (FMD), there is no systematic approach to demonstrate the regional relevance of these products against the specific serotypes and strains that circulate in endemic countries in Africa and Asia. Failure to adopt independent testing of FMD vaccines has contributed to poor trust in their quality and a lack of investment in vaccination programmes. Therefore, a reference antigen panel representing four serotypes, tailored for East Africa, has been established and used to measure FMDV-specific antibody responses in cattle after administration of FMD vaccines commercially available in the region. This revealed inconsistencies and gaps in cross-neutralisation responses that are evident for some vaccines even after giving booster doses. It is concluded that the East Africa reference antigen panel can be used to evaluate FMD vaccine potency and drive up vaccine quality. Further panels could be developed and deployed for other endemic regions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Ferrari G., Paton, D., Duffy, S., Bartels, C. & Knight-Jones, T. Foot and mouth disease vaccines and post vaccination guidelines (eds. Metwally, S., & Munstermann, S.) (FAO & WOAH, 2016).
Grants and funding
- SE2945 and SE1131/UK Department of the Environment, Food and Rural Affairs
- SE2945 and SE1131/UK Department of the Environment, Food and Rural Affairs
- SE2945 and SE1131/UK Department of the Environment, Food and Rural Affairs
- SE2945 and SE1131/UK Department of the Environment, Food and Rural Affairs
- SE2945 and SE1131/UK Department of the Environment, Food and Rural Affairs
- SE2945 and SE1131/UK Department of the Environment, Food and Rural Affairs
- SE2945 and SE1131/UK Department of the Environment, Food and Rural Affairs
- SE2945 and SE1131/UK Department of the Environment, Food and Rural Affairs
- SE2945 and SE1131/UK Department of the Environment, Food and Rural Affairs
- via the European Commission for the Control of Foot-and-Mouth Disease (EuFMD)/The European Union
- via the European Commission for the Control of Foot-and-Mouth Disease (EuFMD)/The European Union
- BB/X011038/1, BB/X011046/1, BBS/E/PI/230002C and BBS/E/PI/23NB0004/Biotechnology and Biological Sciences Research Council (BBSRC) of the United Kingdom
- Twinning Project between The Pirbright Institute and the Pan African Veterinary Vaccine Centre of the African Union/World Organisation for Animal Health
- Twinning Project between The Pirbright Institute and the Pan African Veterinary Vaccine Centre of the African Union/World Organisation for Animal Health
- Twinning Project between The Pirbright Institute and the Pan African Veterinary Vaccine Centre of the African Union/World Organisation for Animal Health
- Twinning Project between The Pirbright Institute and the Pan African Veterinary Vaccine Centre of the African Union/World Organisation for Animal Health
- Twinning Project between The Pirbright Institute and the Pan African Veterinary Vaccine Centre of the African Union/World Organisation for Animal Health
- Twinning Project between The Pirbright Institute and the Pan African Veterinary Vaccine Centre of the African Union/World Organisation for Animal Health
- Twinning Project between The Pirbright Institute and the Pan African Veterinary Vaccine Centre of the African Union/World Organisation for Animal Health
LinkOut - more resources
Full Text Sources
Other Literature Sources

