Oral 8-aminoguanine against age-related retinal degeneration
- PMID: 40419664
- PMCID: PMC12106806
- DOI: 10.1038/s42003-025-08242-1
Oral 8-aminoguanine against age-related retinal degeneration
Abstract
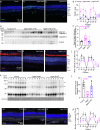
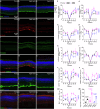
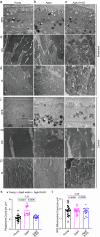
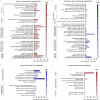
Vision decline in the elderly, often due to retinal aging, predisposes individuals to pathologies like age-related macular degeneration. Currently, there are few effective oral treatments for this condition. Our study introduces an oral agent, 8-aminoguanine (8-AG), which targets age-related retinal degeneration using an aged Fischer 344 rat model. When administered in drinking water at a low dose for 8 weeks starting at 22 months of age, 8-AG significantly preserves retinal structure and function, as evidenced by increased retinal thickness, enhanced photoreceptor integrity, and improved electroretinogram responses. 8-AG reduces apoptosis, oxidative damage, and microglial/macrophage activation in aging retinae. 8-AG also mitigates retinal inflammation at transcriptional and cytokine levels. Extending treatment to 17 weeks further amplifies these protective effects. Given its efficacy in various disease models, 8-AG shows great promise as an anti-aging compound with the potential to mitigate common hallmarks of aging.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Chen Y. is a co-inventor of patents and pending patents: US11,744,826 and 11,191,752. Jackson E. is a co-inventor on issued US patents 10,729,711 and 11,103,526. Jackson E., Birder L., and Wolf-Johnston A. are co-inventors on pending patents US17/434,894, EPO21792357.2, and PCT/US2022/042471. Jackson E., Birder L., Wolf-Johnston A., and Chen Y. are co-inventors on the pending patent US17/799,546, which is associated with this study.
Figures



Update of
-
Oral 8-aminoguanine against age-related retinal degeneration.Res Sq [Preprint]. 2024 May 6:rs.3.rs-4022389. doi: 10.21203/rs.3.rs-4022389/v1. Res Sq. 2024. Update in: Commun Biol. 2025 May 26;8(1):812. doi: 10.1038/s42003-025-08242-1. PMID: 38765984 Free PMC article. Updated. Preprint.
References
-
- Gao, H. & Hollyfield, J. G. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci.33, 1–17 (1992). - PubMed
-
- Curcio, C. A., Millican, C. L., Allen, K. A. & Kalina, R. E. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest. Ophthalmol. Vis. Sci.34, 3278–3296 (1993). - PubMed
-
- Fleckenstein, M. et al. Age-related macular degeneration. Nat. Rev. Dis. Prim.7, 31 (2021). - PubMed
-
- Wong, W. L. et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob. Health2, e106–e116 (2014). - PubMed
MeSH terms
Substances
Grants and funding
- R01 AG056944/AG/NIA NIH HHS/United States
- R01 EY033049/EY/NEI NIH HHS/United States
- R01DK135076/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- R01 EY026891/EY/NEI NIH HHS/United States
- R01 DK135076/DK/NIDDK NIH HHS/United States
- R01 EY030991/EY/NEI NIH HHS/United States
- R01EY026891/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY032482/EY/NEI NIH HHS/United States
- R01EY032482/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01AG056944/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- Unrestricted grant/Research to Prevent Blindness (RPB)
- U01 EY034711/EY/NEI NIH HHS/United States
- R01EY030991/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- S10 RR019003/RR/NCRR NIH HHS/United States
- R01EY033049/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01HL109002/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- U01EY034711/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 CA256068/CA/NCI NIH HHS/United States
- P30EY08098/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- 1S10RR019003/U.S. Department of Health & Human Services | NIH | National Center for Research Resources (NCRR)
- R01 HL109002/HL/NHLBI NIH HHS/United States
- Medical Student Eye Research Fellowship/Research to Prevent Blindness (RPB)
- P30 EY008098/EY/NEI NIH HHS/United States
- R01CA256068/U.S. Department of Health & Human Services | NIH | NCI | Division of Cancer Epidemiology and Genetics, National Cancer Institute (National Cancer Institute Division of Cancer Epidemiology and Genetics)
LinkOut - more resources
Full Text Sources
Medical

