HaloPROTAC3 does not trigger the degradation of the halotagged parasitophorous vacuole membrane protein UIS4 during Plasmodium liver stage development
- PMID: 40419780
- PMCID: PMC12106710
- DOI: 10.1038/s41598-025-98257-9
HaloPROTAC3 does not trigger the degradation of the halotagged parasitophorous vacuole membrane protein UIS4 during Plasmodium liver stage development
Abstract
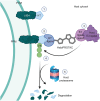
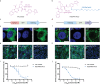
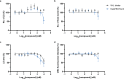
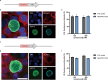
Targeted protein degradation (TPD) is a novel strategy for developing therapeutics against pathogens. Prior to causing malaria, Plasmodium parasites replicate within hepatocytes as liver stages, surrounded by a parasitophorous vacuole membrane (PVM). We hypothesized that TPD can be employed to trigger host-driven degradation of essential liver stage PVM proteins and lead to parasite death. To explore this, we took advantage of the proteolysis-targeting-chimera HaloPROTAC3, a molecule that recruits the host von Hippel-Lindau (VHL) E3 ligase to the HaloTag (HT). Parasites expressing HT fused to the host cytosol-exposed domain of the PVM protein UIS4 (UIS4-HT) were generated in Plasmodium berghei and Plasmodium cynomolgi, but only P. berghei UIS4-HT enabled productive liver stage infection experiments in vitro. Although HaloPROTAC3 triggered the degradation of HT proteins in host cells, it had no impact on the survival of P. berghei UIS4-HT liver stages. Furthermore, HaloPROTAC3 bound to P. berghei UIS4-HT but did not recruit VHL or trigger ubiquitination of the PVM. Overall, although this study did not establish whether host-driven TPD can degrade Plasmodium PVM proteins, it highlights the challenges of developing TPD approaches against novel targets and offers insights for advancing this therapeutic strategy against pathogens.
Keywords: Hypnozoite; Induced proximity; Infectious disease; Malaria; Schizont; Targeted protein degradation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: M.L., A.P., L.T., A.A.L., M.E.F., J.R.K., M.S., A.T., S.H., C.M., E.L.F., D.M., B.N., Z.T., S.A.M., A.H. and G.M. were employed by and/or shareholders of Novartis Pharma AG during this study. All other authors have no competing interest in this study. Ethical approval: Female 6-8-week-old C57BL/6 (Jackson Laboratory) and Swiss Webster (Charles River Laboratories) mice were maintained in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) at Novartis AG. Experiments were conducted in accordance with animal protocols approved by the Institutional Animal Care and Use Committee (IACUC, Novartis). Mice were anesthetized using isoflurane (3% isoflurane and 2 L/min oxygen) prior to retro-orbital injections and euthanized with CO2 followed by either cervical dislocation or bilateral thoracotomy for terminal blood collection. Euthanasia methods were in accordance with the recommendations set forth in the latest Panel Report of the American Veterinary Medical Association guidelines on euthanasia or pre-approved by the IACUC and the attending veterinarian. All procedures involving NHPs were reviewed and approved by the IACUC of OHSU / ONPRC or UGA. Both OHSU / ONPRC and UGA are certified by AAALAC International. For experiments performed at UGA, male Japanese macaques (Macaca fuscata) were obtained from ONPRC, transported to UGA for use in malaria-related studies, and underwent quarantine procedures upon arrival. For experiments performed at OSHU, Japanese macaques and rhesus macaques (Macaca mulatta) are purpose-bred at ONPRC. Prior to use, all NHPs underwent thorough physical exams and blood work to confirm their suitability for malaria-related studies. All NHPs were socially housed when compatible animals were available and when it did not interfere with the study goals and were provided environmental enrichment consisting of daily feeding enrichment, provision of manipulanda, and physical enrichment as described in the Guide for the Care and Use of Laboratory Animals. All procedures, including blood collections, inoculation procedures, parasitemia monitoring, sedation and analgesia protocols, were reviewed and approved by the IACUC prior to the beginning of an infection and were followed accordingly. The authors confirm that this study is reported in accordance with the ARRIVE guidelines.
Figures



References
-
- World Health Organization. Antimicrobial resistance: global report on surveillance (WHO, 2014).
-
- World Health Organization. World Malaria Report (WHO, 2023).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

