Diagnostic accuracy of artificial intelligence-based multi-spectrum analysis for molecular fingerprint detection of SARS-CoV-2
- PMID: 40419915
- PMCID: PMC12114054
- DOI: 10.1097/MD.0000000000041928
Diagnostic accuracy of artificial intelligence-based multi-spectrum analysis for molecular fingerprint detection of SARS-CoV-2
Abstract
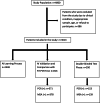
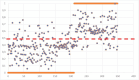
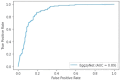
Reverse transcription-polymerase chain reaction (RT-PCR) is the reference standard for COVID-19 diagnosis, but the need for rapid, reproducible, and cost-effective diagnostic tools remains. This study investigated the diagnostic performance of a novel artificial intelligence-based multispectrum analysis (MSA, AP23) technique that detects the biomolecular fingerprint of severe acute respiratory syndrome coronavirus 2. A prospective, double-blinded observational design was used, involving 3614 volunteers. The artificial intelligence was trained with 2448 samples, validated with 816, and tested against RT-PCR using a blinded set of 350 samples. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated. During validation, MSA achieved 88.4% sensitivity, 88.76% specificity, 86.77% positive predictive value, and 90.18% negative predictive value. In the blinded comparison phase, these values were 81.73%, 81.99%, 75.16%, and 87.81%, respectively, with an area under the receiver operating characteristic curve of 0.89. These findings suggest that MSA offers reliable diagnostic performance and may be a promising alternative to RT-PCR in COVID-19 diagnosis. The study was registered on ClinicalTrials.gov (NCT04860895).
Keywords: SARS-CoV-2; artificial intelligence; molecular fingerprint; multi-spectrum analysis.
Copyright © 2025 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures
Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2022 May 16;5(5):CD013639. doi: 10.1002/14651858.CD013639.pub5. Cochrane Database Syst Rev. 2022. PMID: 35575286 Free PMC article.
-
Evaluation of the Seegene Allplex™ RV master assay for one-step simultaneous detection of eight respiratory viruses in nasopharyngeal specimens.J Virol Methods. 2025 Jan;331:115042. doi: 10.1016/j.jviromet.2024.115042. Epub 2024 Oct 9. J Virol Methods. 2025. PMID: 39384158
References
-
- WHO. Coronovirus (COVID-19) dashboard. https://covid19.who.int/. Accessed August 9, 2022.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

