Time to treatment in pediatric patients with repeated episodes of status epilepticus
- PMID: 40420060
- PMCID: PMC12105332
- DOI: 10.1186/s12883-025-04200-w
Time to treatment in pediatric patients with repeated episodes of status epilepticus
Abstract
Objective: To compare pediatric patients who presented with repeated status epilepticus episodes to patients with a single episode of status epilepticus and identify distinguishing clinical factors.
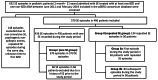
Methods: Retrospective analysis of a multicenter, prospective observational cohort of pediatric patients with status epilepticus between 2011 and 2019.
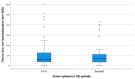
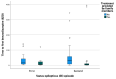
Results: Out of 504 status epilepticus episodes in 420 patients, 50 patients (10.3%) had repeated episodes of status epilepticus. The only predictor of repeated status epilepticus was a prior diagnosis of epilepsy. There was no difference in time to treatment with the first benzodiazepine in patients presenting with their first status epilepticus episode compared to their second status epilepticus episode [median 10 (interquartile range 5-30) vs. 14 (4.5-52.5) minutes; (p = 0.24)] or in time to treatment with the first non- benzodiazepine anti-seizure medication (ASM) [61 (37-125) vs. 71 (34.5-117.5) minutes; p = 0.61]. In patients with repeated status epilepticus episodes with onset outside the hospital, the percentage of patients treated by caregivers did not improve between the first and second status epilepticus episode (61% vs. 60%, p = 0.56). However, the time to first benzodiazepine was shorter in patients treated by caregivers compared to those who were not [5 (0-25) vs. 55 (41-120) minutes; p < 0.001].
Conclusions: Time to treatment with benzodiazepine and non-benzodiazepine ASM in patients with repeated episodes of status epilepticus does not improve for a second episode of status epilepticus, suggesting additional opportunities for intervention and teaching.
Keywords: Anti-seizure medications; Benzodiazepines; Refractory status epilepticus; Status epilepticus; Treatment delay.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The research protocol was approved by the institutional review boards of all participating institutions, including Boston Children’s Hospital (IRB P-00001207), and written informed consent was obtained from all participants, parents, or guardians. Consent for publication: Not applicable. Competing interests: Dr. Jennifer Gettings receives research support from NIH. Dr. J. Nicholas Brenton receives research support from NIH. He has served as a consultant for Cycle Pharmaceuticals and the Institute for Advanced Clinical Trials (I-ACT) for Children on a Novartis-sponsored project. Dr. Adam Ostendorf receives research support from NIH Dr. Edward Novotny was on the Advisory Board for Longboard Pharmaceuticals, Inc. Dr. Mark Wainwright is a member of the Clinical Advisory Board for Sage Therapeutics. Dr. Tobias Loddenkemper receives research support from NIH, Epitel, Sumitomo, and the Epilepsy Research Fund. He received past research support from Upsher Smith, Proximagen, MIKU, and UCB. Dr. Tobias Loddenkemper is part of patent applications to detect and predict clinical outcomes, and to detect, manage, diagnose, and treat neurological conditions, epilepsy, and seizures. Some of Dr. Tobias Loddenkemper’s trainees received salary support from international foundations/societies and academic centers while working in his laboratory. Dr. Marina Gaínza-Lein was previously funded by the Epilepsy Research Fund.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

