Evodiamine induces ferroptosis in prostate cancer cells by inhibiting TRIM26-mediated stabilization of GPX4
- PMID: 40420092
- PMCID: PMC12105283
- DOI: 10.1186/s13020-025-01130-0
Evodiamine induces ferroptosis in prostate cancer cells by inhibiting TRIM26-mediated stabilization of GPX4
Abstract
Background: Prostate cancer is a major global health challenge, characterized by high morbidity and mortality rates. Traditional treatment options, including androgen deprivation therapy and chemotherapy, often lead to drug resistance. In recent years, natural compounds have garnered attention for their potential therapeutic effects. Evodiamine, a bioactive alkaloid from Evodia rutaecarpa, has demonstrated promising anti-cancer properties in various malignancies, including oral squamous cell carcinoma, breast, colorectal, and ovarian cancers. This study explores the efficacy of evodiamine in prostate cancer cells and investigates the mechanisms underlying evodiamine-induced cell death.
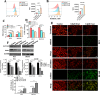
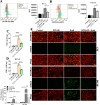
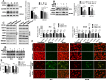
Methods: To investigate the effects of evodiamine on prostate cancer cells, various cell lines, including both castration-sensitive and castration-resistant variants, were treated with different concentrations of evodiamine for various durations. Cell viability, proliferation, invasion ability, and colony formation were assessed using the CCK8 assay, EdU assay, 3D matrigel drop invasion assay, and colony formation assay, respectively. The effects of evodiamine on apoptosis were analyzed using FACS, Hoechst staining, and Western blot. To evaluate its effects on ferroptosis, malondialdehyde (MDA) and glutathione (GSH) assay kits, as well as DCFH-DA and the lipid peroxidation sensor BODIPY™ 581/501 C11 fluorescent probes, were employed. The molecular mechanisms through which evodiamine regulates GPX4 protein instability were investigated using Western blot and TRIM26 ectopic expression. Additionally, a mouse xenograft model derived from DU145 cells was established to evaluate the in vivo effects of evodiamine and its molecular mechanisms, utilizing hematoxylin and eosin (H&E) staining, immunohistochemistry (IHC), and Western blot analysis.
Results: Evodiamine significantly suppressed cell viability, proliferation, invasion, and colony formation in prostate cancer cells. Importantly, evodiamine-induced cell death in the PC3 and DU145 cell lines was independent of apoptosis pathway. Instead, evodiamine increased reactive oxygen species (ROS) production, lipid ROS levels and MDA levels, while decreasing GSH levels, indicating the induction of ferroptosis. The key role of ROS in evodiamine-induced ferroptosis was further confirmed by the partial reversal of cell death upon treatment with the ROS scavenger N-acetylcysteine (NAC). Mechanistically, evodiamine induced ferroptosis by destabilizing GPX4 protein in a TRIM26-dependent manner. Moreover, in vivo studies demonstrated that evodiamine significantly inhibited tumor growth and induced ferroptosis in tumor cells, highlighting its therapeutic potential.
Conclusion: This study demonstrates that evodiamine exerts potent antitumor effects against prostate cancer through inhibiting TRIM26-mediated stabilization of GPX4 protein and triggering ferroptosis. These findings suggest that evodiamine, a natural product derived from traditional Chinese medicine, could be a promising therapeutic agent for prostate cancer.
Keywords: Evodiamine; Ferroptosis; GPX4; Prostate cancer; TRIM26.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: In this study, all animal experiment and procedures have been approved and performed in accordance with the Animal Care Welfare Committee of Lanzhou University Second Hospital Ethics approval and consent to participate (D2024-011). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures





References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022 GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clinic. 2024;74(3):229–63. - PubMed
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA A Cancer Clinic. 2024;74(1):12–49. - PubMed
-
- Miyahira AK, Soule HR. The 30th annual prostate cancer foundation scientific retreat report. Prostate. 2024;84(14):1271–89. - PubMed
-
- Kaur P, Shankar E, Gupta S. EZH2-mediated development of therapeutic resistance in cancer. Cancer Lett. 2024;586: 216706. - PubMed
Grants and funding
- 82203167/National Natural Science Foundation of China
- 82460722/National Natural Science Foundation of China
- GSWSQNPY2024-12/gansu provincial health industry science and technology innovation project
- CY2022-MS-A09/cuiying scientific and technological innovation program of lanzhou university second hospital
- CY2023-MS-A05/cuiying scientific and technological innovation program of lanzhou university second hospital
LinkOut - more resources
Full Text Sources

