Development of human targeted extracellular vesicles loaded with shRNA minicircles to prevent parkinsonian pathology
- PMID: 40420149
- PMCID: PMC12105355
- DOI: 10.1186/s40035-025-00484-7
Development of human targeted extracellular vesicles loaded with shRNA minicircles to prevent parkinsonian pathology
Abstract
Background: Neurological disorders are the second leading cause of death and the leading cause of disability in the world. Thus, the development of novel disease-modifying strategies is clearly warranted. We have previously developed a therapeutic approach using mouse targeted rabies virus glycoprotein (RVG) extracellular vesicles (EVs) to deliver minicircles (MCs) expressing shRNA (shRNA-MCs) to induce long-term α-synuclein down-regulation. Although the previous therapy successfully reduced the pathology, the clinical translation was extremely unlikely since they were mouse extracellular vesicles.
Methods: To overcome this limitation, we developed a source of human RVG-EVs compatible with a personalized therapy using immature dendritic cells. Human peripheral blood monocytes were differentiated in vitro into immature dendritic cells, which were transfected to express the RVG peptide. RVG-EVs containing shRNA-MCs, loaded by electroporation, were injected intravenously in the α-synuclein performed fibril (PFF) mouse model. Level of α-synuclein, phosphorylated α-synuclein aggregates, dopaminergic neurons and motor function were evaluated 90 days after the treatment. To confirm that EVs derived from patients were suitable as a vehicle, proteomic analysis of EVs derived from control, initial and advanced Parkinson's disease was performed.
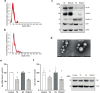
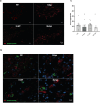
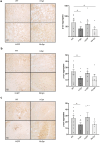
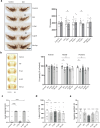
Results: The shRNA-MCs could be successfully loaded into human RVG-EVs and downregulate α-synuclein in SH-SY5Y cells. Intravenous injection of the shRNA-MC-loaded RVG-EVs induced long-term downregulation of α-synuclein mRNA expression and protein level, decreased α-synuclein aggregates, prevented dopaminergic cell death and ameliorated motor impairment in the α-synuclein PFF mouse model. Moreover, we confirmed that the EVs from PD patients are suitable as a personalized therapeutic vehicle.
Conclusion: Our study confirmed the therapeutic potential of shRNA-MCs delivered by human RVG-EVs for long-term treatment of neurodegenerative diseases. These results pave the way for clinical use of this approach.
Keywords: Gene therapy; Human targeted extracellular vesicles; Parkinson’s disease; ShRNA minicircles; α-Synuclein.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All studies involving human samples were approved by the Institutional Ethical Committee (CEImLar, protocol number: P.I.374). All animal studies involving mice followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the ethical committee on animal welfare at our institution (Center for Biomedical Research of La Rioja, ref LAE-03). Consent for publication: Not applicable. Competing interests: LAE, EC and MI have filled a patent application related to the work in this paper. The remaining authors declare no competing interests.
Figures



References
-
- Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T. The Incidence of Parkinson’s disease: A systematic review and meta-analysis. Neuroepidemiology. 2016;46(4):292–300. - PubMed
-
- Lee VM, Trojanowski JQ. Mechanisms of Parkinson’s disease linked to pathological α-synuclein: new targets for drug discovery. Neuron. 2006;52(1):33–8. - PubMed
-
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501–3. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

