Sequential nanoparticle therapy targeting neutrophil hyperactivation to prevent neutrophil-induced pulmonary fibrosis
- PMID: 40420186
- PMCID: PMC12105360
- DOI: 10.1186/s12951-025-03421-y
Sequential nanoparticle therapy targeting neutrophil hyperactivation to prevent neutrophil-induced pulmonary fibrosis
Abstract
Background: Pulmonary fibrosis, a major complication of severe COVID-19 and post-acute sequelae of SARS-CoV-2 infection (PASC), is driven by excessive neutrophil activation and the formation of neutrophil extracellular trap (NET).
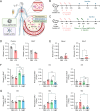
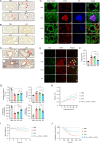
Results: This study presents a sequential nanoparticle-based therapy combining DNase-I-loaded polydopamine nanoparticles (DNase-I@PDA NPs) with Sivelestat-encapsulated PLGA nanoparticles (Siv@PLGA NPs) to target both NETs and neutrophil elastase (NE) activity. DNase-I@PDA NPs were aerosolized to the lungs, facilitating NET clearance and reducing the fibrotic microenvironment, followed by intravenous administration of Siv@PLGA NPs to inhibit NE activity and prevent neutrophil hyperactivation. In a murine model of lipopolysaccharide (LPS)-induced pulmonary fibrosis, this dual approach significantly decreased fibrotic lesions, collagen deposition, and myofibroblast activation. Notably, treatment with the nanoparticles led to substantial improvements in pulmonary function. In neutrophils isolated from COVID-19 patients, the combined nanoparticle therapy reduced circulating cell-free DNA, NET, NE, and myeloperoxidase (MPO) levels, while enhancing neutrophil viability and reducing inflammatory responses.
Conclusions: These findings highlight the efficacy of DNase-I@PDA NPs and Siv@PLGA NPs in addressing both acute inflammation and chronic fibrosis by simultaneously targeting NET formation and neutrophil hyperactivation. This dual nanoparticle therapy represents a promising clinical strategy for treating COVID-19-associated pulmonary complications, including PASC, by preventing long-term fibrotic progression and promoting lung recovery.
Keywords: Aerosolized drug delivery; Chronic inflammation; Neutrophil extracellular traps; Post-acute sequelae of SARS-CoV-2 (PASC); Pulmonary fibrosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee of Yeungnam University Medical Center, and all participants provided consent to participate (YUH 2020-03-057 and 2020-05-031-001). This research received approval from the Ethics Committee of Sungkyunkwan University, and all procedures with animal models followed established ethical standards (IACUC No.: 202111291). Consent for publication: Consent for publication is not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

